-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Zoology
2012; 2(3): 7-13
doi: 10.5923/j.zoology.20120203.01
Age, Growth and Reproduction of the Female Kutum, Rutilus kutum (Kamensky, 1901) (Teleostei: Cyprinidae), in Gorgan-Rud Estuary, Northern Iran
Yazdan Keivany, Parviz Zare, Latif Kalteh
Department of Fisheries, Faculty of Natural Resources, Isfahan University of Technology, Isfahan 84156-83111, Iran
Correspondence to: Yazdan Keivany, Department of Fisheries, Faculty of Natural Resources, Isfahan University of Technology, Isfahan 84156-83111, Iran.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Fishes in the eastern part of the Caspian Sea experience a higher temperature and salinity, compared to those in the western part. This study was conducted on 66 specimens of female kutum, Rutilus kutum, from a migratory population to Gorgan-Rud River during March-May to compare it with the most studied western populations and to study the effects of these ecological factors on biology of kutum. The population comprised six age groups. The 5+ and 6+ age groups were dominant in the studied population. The sex ratio was 1:1.5 (M:F) that was significantly different from 1:1 ratio (p<0.05). The slope (b) of the fork length-weight regression line indicated an isometric growth (p>0.05). Relative and instantaneous growth rates decreased with age. The mean value of condition factor was 1.50 which was not correlated with age. Absolute fecundity varied from 13494 (7 years old) to 115177 eggs (8 years old). The average absolute and relative fecundity were 70300 and 51, respectively. The relationships of fecundity (F) with fork length (FL) was better stated as a power and with total weight (W), ovary weight (Wo) and age (t) as linear equations. Fecundity with FL, W, Wo and age had positive and high correlations. The relationships of ovary weight with total weight and fork length were as a linear function. Egg diameter varied from 158 µm to 251 µm (mean=194±17). Gonadosomatic index (GSI) varied from 4.92 to 29.05 (19.42±4.53) during the reproduction period and its peak determined to be in the last week of April. It is concluded that these ecological factors could highly affect the kutum populations and compared to the western populations, the eastern population differed in a lower b value, a lower ratio of male:female, a lower absolute and relative fecundity and a higher egg diameter. This extends our knowledge on the biology of kutum populations in different areas of the Caspian Sea.
Keywords: Ageing, Caspian Sea, Cypriniformes, Fecundity, Gorgan-Rud estuary, Growth
Article Outline
1. Introduction
- The kutum or mahi-ye sefid (= white fish, in Farsi), Rutilus kutum (Kamensky, 1901), is one of the commercially most important and valuable cyprinids of the Caspian Sea and distributed from Atrek River to Gorgan Bay in the southern part of the sea[1,2]. This fish is a true migratory or anadromous species which enters the rivers for spawning. Many workers[3-6] recognize two forms for this species; autumn form which enters the rivers in October to February, and spring form which enters the rivers in March to April. Recently, due to the destruction of rivers, natural spawning of the fish has reduced significantly, on the other hand, millions of hatchery reared fry are released annually[7]. Catching rate of the fish has fluctuated from 3500 to 8500 metric tons during 1991-97 in Iran and is increasing thanks to mass production and release of hatchery reared fry[8].The catch rate of the fish in 2002 was 450 on Golestan coasts and 30-32 tons in Gorgan-Rud estuary which comprises about 6.8% of the province total catch. The total catch was 6000-8000 metric tons in Iranian part of the Caspian Sea in year 2004. However, the fish stock biomass was estimated as about 25000 tons[9].Studying age, growth, and reproduction of fishes is necessary for their sustainable exploitation, preservation, successful propagation, and management. Despite the economic importance of the fish, its biology in Gorgan region is not well documented and most of the available information is from the western coast[1,10-17]. Some of these works include studied on fertility[1], ecology[2], spawning[6], fecundity[10], fishing and reproduction[11,16], feeding[12] and age structure[15] of kutum. Since the physical and chemical conditions of Gorgan-Rud estuary and the eastern region are different from those of the western region (e.g., higher temperature and salinity), in the present work, we studied the age, growth, fecundity and their relationships in the females from Gorgan-Rud estuary during its breeding season to compare them to those of the western region and to study the effects of these ecological factors on kutum.
2. Materials and Methods
2.1. Sampling
- Amongst 802 specimens of kutum caught in March-May, 66 ripe females at stages 4-7 of maturity were sorted out weekly. A seine net with 28 mm sac mesh, 1200 m length and 10-12 m height, about 2500 m from the shore in the middle of the estuary was used. Gorgan-Rud estuary is located in northern Golestan province and in the Caspian Sea basin. The specimens were fixed in 10% formalin and transferred to lab for further studies.
2.2. Ageing
- Age determination was carried out using 10-15 scales collected from above the lateral line and anteroventral part of the dorsal fin as suggested by[18-20].
2.3. Biometry and Growth
- Total length, fork length, and standard lengths were measured at the nearest 1 mm. The total body weight was measured at the nearest 0.1 g. The following formula was used to estimate the weight–length relationship[21,22] W= aFLb. The relative growth based on length and weight was calculated by the following formulae[20] Where RFL= relative fork length and RW= relative weight.
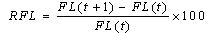 | (1) |
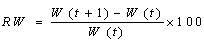 | (2) |
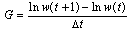 | (3) |
 | (4) |
2.4. Reproduction
- The ovaries were taken out carefully from the abdomen and weighed using a digital scale to the nearest 0.1g and kept in 7% formalin. The gonadosomatic Index (GSI)[29] or maturity index, an indirect method for estimating spawning period[30], was calculated for each day, by the following formula, where Gw= Gonad weight and TW= total length:
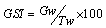 | (5) |
 | (6) |
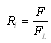 | (7) |
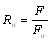 | (8) |
2.5. Statistical Analyses
- To determine the best fit for the relationships between fecundity and fork length, total weight and age, linear, binomial, power, and exponential equations were viewed[20,31]. Ratio of female:male in the clutch was determined and tested by Chi-Square. The t-test used for comparing means. All the calculations were carried out in Minitab 13.3 and SPSS 18.0 computer programs.
3. Results
3.1. Ageing
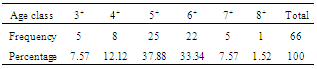
- The females were divided into 6 age groups; 3+ to 8+ (Table 1). 1+, 2+ and higher than 8+ groups were not observed. In this study, 5+ and 6+ age groups were the dominant fishes. The oldest specimen was an 8 year old female. Amongst the 802 landed specimens, the sex ratio was 1:1.5 (Female:Male); 39% female versus 61% males and were significantly different (p<0.05) from 1:1 ratio.
|
3.2. Biometry and Growth
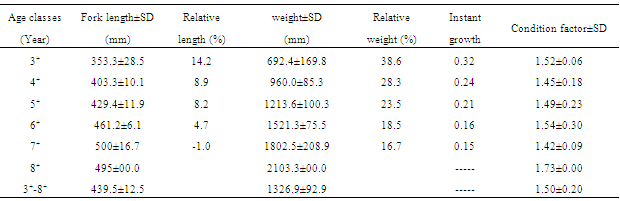
- The average total, fork and standard length were 483.8, 439.5 and 417.2 mm, respectively. The range for the lengths is shown in Table 2. The average weight was 1326.9g. The range was between 468.6 (in a 345 mm fish) and 2281.7g (in a 490 mm fish). As shown in Fig. 1, the frequency of length class 444-473 mm is the highest. The relationship between fork and standard lengths was linear (Table 2). The weight-length relationship in the studied fish was as W = 0.019 FL2.930 (r2 = 0.886) (Fig 2). The mean weight of mature female is highly correlated with length. The b value (2.930) was not significantly different from 3 which is indicative of an Isometric growth (P>0.05).
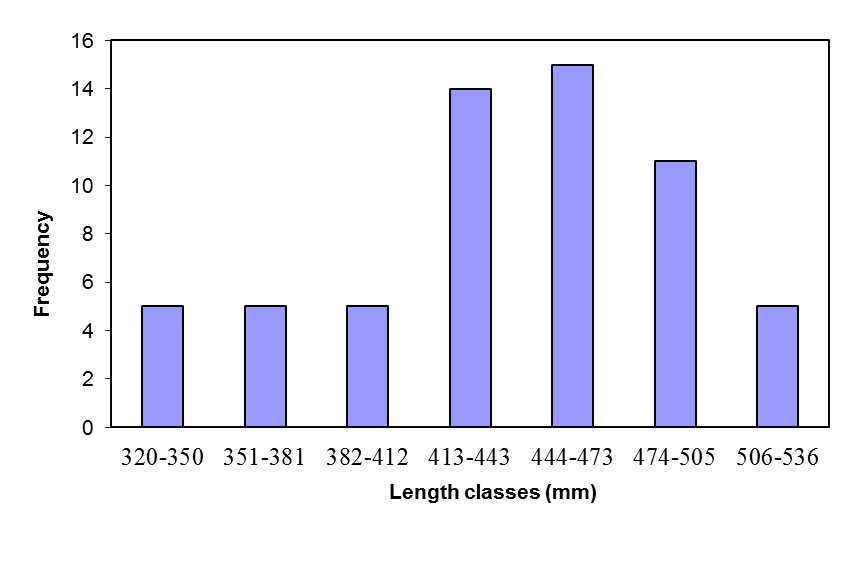 | Figure 1. Frequency of different length classes of female kutum in Gorgan-Rud estuary |
 | Figure 2. Weight-length relationship of female kutum in Gorgan-Rud estuary |
|
|
|
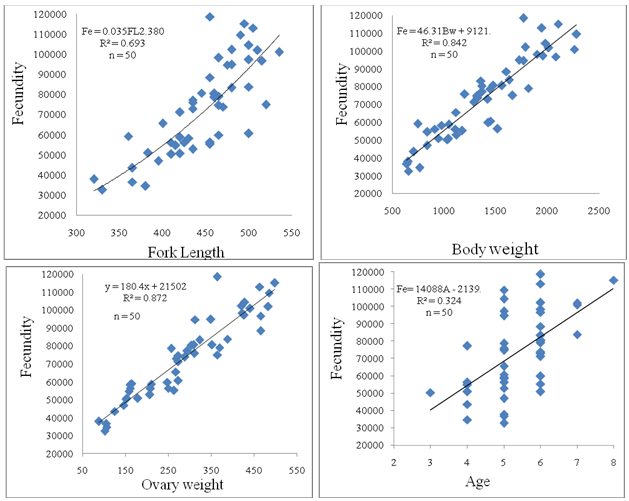 | Figure 3. Relationship of fecundity with fork length, body weight, ovary weight and age in kutum in Gorgan-Rud estuary |
3.3. Reproduction
- The mean absolute fecundity was 70300±25790, with a range of 13494 in 7 year old fish to 115177 in 8 year old fish. The relative fecundity was 50 per 1 g of body weight and 150 per 1 mm of fork length. The fecundity showed highly positive correlations with length, weight, and ovary weight (P<0.05), but a low correlation with age (Fig 3). The equations for these correlations are as below:Fe= 0.035 FL2.380 (r2 =0.69)Fe= 46.31 WB + 9121 (r2 =0.84)Fe= 180.4 WO + 21502 (r2 =0.87)Fe= 14088 t – 2139 (r2 =0.32)The relationships of ovary weight with total weight and fork length is as follow:Wo= - 23.5 + 0.22 W (r = 0.90)Wo= - 634.4 + 2.052 FL (r = 0.67)It appears that the ovary weight more correlated with body weight than fork length. The egg diameter was 158-251µ with an average of 194±17 and positively correlated with length, weight and age. Egg diameter increases with age. The gonadosomatic index varied between 4.92 and 29.05 in March–May and increased with age; the highest mean being in 8+ and the lowest in 3+ age groups (Table 4). The highest mean of GSI was in the last week of April, indicating the peak of the spawning period.
4. Discussion
- In this study the age of the female kutum migrating to Gorgan-Rud river varied from 3+ to 8+ years, however, that could be due to using a 280 mm mesh size net which prevented catching age groups under 3 years. About 75% of the specimens were at the age of 5 and 6. The maximum age reported for this species is 10 years. References[13,32] in the same area found a similar age groups as in ours. Reference[33] reported the age groups for fish caught by the coastal seines in Guilan and Mazandaran provinces (western part), between 1+ to 8+; 12.8% of them being in the immature 1+ and 2+ age groups. Some of the 3+ individuals were also immature and comprised 6.7% of the total catch while in the last decades the 5+ and 6+ age groups were the dominant groups[34,35]. References[15,16] reported age classes 3-7 in the western part of the Caspian Sea.The value of b in the length-weight equation was 2.930 indicative of an isometric growth. This results differ from that of[13] who calculated that as 3.1[33], as 2.86[15] and as 3.04 and concluded an allometric growth for this species in the western region. It is recommended to calculate the two sexes separately. The b value often equals 3 and ranges between 2.5 and 4.5. In fishes, like other animals, the weight fluctuation is higher than length and follows a power equation. Variation in the b value also depends on the different developmental and growth stages, age differences, ripeness, species, geographic status, environmental conditions, fishing season, stomach evacuity of the fish, diseases, and parasites[19,20]. Reference[3] showed that the slope of the length-weight regression line is not fixed and would change significantly by factors affecting the fish growth. Variation in growth is considered as an adaptation to different ecological conditions such as temperature and food quality and quantity[29,37].The condition factor showed no regular trend with the age. In[32], the condition factor increased by age in females, but in our study was irregular and the highest value was for the 8+ age group. The differences in the condition factor might be related to different environmental conditions such as season, food quality and water system (lake vs. river) and interspecific variations. The highest value of instant growth, percentage of relative length and weight, occurred in the 3+ age group (Table 3) and regularly decreased with ageing. In contrary, in[32], the instant growth rate showed a great variation not containing in the growth laws.The sex ratio in the migratory fish was 1:1.5 male to female which may not be indicative of the population in other stages of their life. Reference[32] calculated the ratio as 1:1.6, a figure very close to our findings. Reference[15] reported a ratio of 3.33:1 for the male:female in Tonekabon River, western part of the Caspian Sea. The overall sex ratio in most fishes is close to 1 but it may deviate in different age and size groups. Males are usually dominant in younger age groups because they reach the maturity earlier and have a shorter life span[29].Reference[1] reported the average absolute fecundity as 106505 and the highest value as 198560 (in a 63 cm and 4 kg fish) and the lowest as 36200 (in a 38 cm and 840 g fish) and[38] estimated the absolute fecundity as 74774, both in the western part. The highest absolute fecundity in Gorgan-Rud estuary was 115177. The relative fecundity in Gorgan-Rud estuary was 51 eggs per 1 g of body weight, but it was reported as 49 in Anzali Wetland[1] and 58 in Havigh River[13], both in the western part. The relative fecundity, based on the fork length, was 150 eggs per 1 cm. Reference[1] reported this figure as 169 and[13] as 208. Weight fluctuation due to environment changes, season, spawning immigration, parasites and diseases can affect the fecundity and thus, the relative fecundity based on length should be used[39]. The minimum number of eggs per 1 g of eggs was 178 and the maximum was 430 and 208 on average. Our result shows a significant difference with[13] results in which he reported the range of egg weight as 205-430 with an average of 304 in the western part. Most researchers such as[41-43] considered the relation between absolute fecundity and length as a third degree power equation. While some others such as[44,45] consider it as a square of length. A linear relationships between fecundity and weight was reported[46,47]. Reference[48] found a linear relationship between the fecundity and weight and concluded that fecundity is more correlated with weight rather than length of the females. However, some researchers argued that the correlation between fecundity and weight is not more than that of between fecundity and length, because the weight changes just before the spawning season[49,50]. Reference[47] believed that in fishes where b is about 3, the relationship is as a power equation.Most researchers reported that the fecundity increases as ovary weight increases[51]. Reference[52] stated that in most fishes the egg number do not change significantly with season, but ovary weight causes increase in their volume and mass that is from consumed food or body tissue. Thus, there is disagreement on the effects of egg size on fecundity raised by[53] and followed by others such as[54]. However, some others such as[55] showed that eggs are not the only factors affecting fecundity, but the differences in fecundity of a species in different regions are related to genetic differences of different subspecies and it is affected by age, size, species, diet, season, environmental conditions and year. In general, bigger fish has a higher fecundity and the correlation of fecundity to weight in most fishes is more than that to length and age[56]. Our results indicated a significant positive correlation between fecundity and length, body and ovary weight. However, it differed in different age groups; older fish had a higher fecundity as reported by[20,31] though some authors did not observe such a correlation[55].These different results regarding fecundity and length, weight and age is determined by different maturity cycles, life span and age structure of the population and may be due to sampling and statistical analysis accuracy. In populations reaching maturity early in their life with almost the same size classes, the relationship is presented in the form of power or exponential equations. In populations reaching maturity in latter stages (when there is no younger fish age groups and higher age classes are well represented in the sample) are shown by logarithmic equation. Where lower age groups are not present, the relation of fecundity with length and age could be linear. For populations with premature condition and a long life cycle and availability of all sizes and ages, the relationships of fecundity with age and length is sigmoid[31]. The results of fecundity-weight analysis is almost the same as it is expected, which is usually linear. Reference[31] showed that in some waters the relationship between fecundity and weight in perch is not linear. In this study, the relationship of fecundity with length is power and with body weight, ovary weight and age is linear. However, the correlation index for fecundity-ovary weight in a power equation was the same as in the linear equation. Reference[32] reported the relationships of fecundity-total length as linear with a correlation index of 0.71.One of the most important factors in reproductive biology is the egg diameter. In the individuals with the same age, the bigger egg is indicative of having more yolk and hence a higher survival rate of the larvae. Our results showed that the eggs size and weight is significantly correlated with the fish length, weight and age; larger and older fish had bigger eggs. Besides, with an increase in fecundity, the size and weight of the eggs increased. The egg diameter ranged between 179-251µ (194±17). Reference[1] reported the egg diameter as 150-250µ (178µ) and[38] as 120-180µ, both in the western part.The gonadosomatic index differed in different ages. Reference[32] reported that to be between 4.14 and 21.26. Studied population of Liagina lake indicated that the GSI is variable in different years probably due to degree of maturity and different fecundity in the same size groups. Our results showed a correlation between ovary weight with body weight and length. Reference[57] showed that GSI is the best sexual activity indicator which is variably correlated with length. The maturity age appears to be 3 as reported by other authors from the same area[32] and Guilan coasts, while was reported as 4 by[13] in cooler regions and probably spawn until the age of 11, but mostly were 3-5 years old. Among our 3+ age group, only one specimen carried eggs. Reference[20] showed that age of maturation depends on several factors such as species, temperature, feeding quality and quantity. Reference[57] stated that lower maturity age reduces the life span. However, selective fishing of larger brooders can introduce large errors in the estimation.
5. Conclusions
- In conclusion, the eastern populations of kutum differ from the western populations in a lower b value, a lower ratio of male:female, a lower absolute and relative fecundity and a higher egg diameter mean. It's been shown that these ecological factors could highly affect the kutum populations in different parts of the Caspian Sea that could be used for fishing, stocking and management purposes.
ACKNOWLEDGEMENTS
- We would like to thank Dr. A.A. Khanipour, Dr. A. Taghavi-Motlagh, Mr. D. Ghaninezhad (Bandar Anzali Fisheries Research Center, Iran), Managers of the Nour Golestan and Shayan Aidin Fishing Cooperatives and Dr. R. Patimar (Gorgan University of Agricultural Sciences and Natural Resources) for their various help. Original version of this template was provided by courtesy of SAP Productions. This study was financially supported by Isfahan University of Technology.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML