-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Zoology
2012; 2(1): 5-7
doi: 10.5923/j.zoology.20120201.02
Gestation Effects on the Hematological and Biochemical Profile of Nubian Ibex (Capra Nubiana)
M.S. AL-Eissa 1, Saad Alkahtani 2
1Department of Biology, Faculty of Science, Hail University, Riyadh 11362, Saudi Arabia
2Departement of Science, Teachers College, King Saud University, Riyadh 11352, Saudi Arabia
Correspondence to: M.S. AL-Eissa , Department of Biology, Faculty of Science, Hail University, Riyadh 11362, Saudi Arabia.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The aim of this study was to investigate effect of gestation periods on thehaematological and biochemical parameters in adult captive wild (Capra nubiana),a group of 21Nubian ibexwas selected for the study. the mean values of the different (RBC) and (WBC) showed significant changes during gestation. (RBC), (PCV) and (Hb) increased while total (WBC) and segmented neutrophils increased after the second gestation period in the second period. The numbers of total leucocytes and segmented neutrophilsincreased after the second period. The concentration of the (AST) was declined during progression of gestation, On the other hand the concentration of (ALT) was increased and arrived maximum concentration at second period of gestation.
Keywords: Nubian Ibex, Pregnancy, Gestation, Haematology, Biochemistry
Cite this paper: M.S. AL-Eissa , Saad Alkahtani , "Gestation Effects on the Hematological and Biochemical Profile of Nubian Ibex (Capra Nubiana)", Research in Zoology , Vol. 2 No. 1, 2012, pp. 5-7. doi: 10.5923/j.zoology.20120201.02.
Article Outline
1. Introduction
- In Saudi Arabia, few systematic records have been made,most of the information (data) was exhibited from brief aerial and ground exploration survey made to locate populations. Among the 15 sites where ibex has been found, major concentrations are observed in the western mountains of the Arabian shield with isolated populations located in the north, north-central and central regions. Only scattered observations have been made in the south (Aleissa M, 2011). There is no accurate population estimation, but the overall numbers are believed to be decreasing in the area where ibex species is not protected. Gestation lasts about five months and the majority of young are born in March ( The Ultimate Ungulate Page (January, 2006) . A litter size of one is usual, but twins and, very rarely, triplets occur ( University of California, San Diego: School of Medicine (January, 2006)). Sexual maturity is reached at two to three years, and offspring then leave their natal herd. Hunting, livestock, extension of the road and other development pressures are fundamentally degrading habitat in Saudi Arabia.lackinformation is found regarding the hematological parameters of Ibex species (Perez´s et al., 2003), However few reports on biochemical baseline information for the genus Capra is available, none is available for the species Capra nubiana. (Perez´s et al. 2003) studied the haemato logical parameters of the Spanish Ibex. The study of the Hematological and biochemical profiles is a vigorous diagnostic assistance. It is well known fact that factors such as breed, sex, age, behavior, handling, physiological changes and the period of the day, can influence the cellular constituents and serum biochemistry of the blood (Roy, 2010).The aim of this study was undertaken to collect and evaluateselected biochemical and hematological parameters of captive Capra nubiana Ibex under the effect of the first, second and third period of pregnancy. Mean values obtained for each hematological variable during the different periods ofpregnancy were compared for each breed to establishreference values during gestation.
2. Materials and Methods
- The animals used in this study were 21 adult pregnant females (aged 4-7 years old), all were clinically normal and healthy Ibex (Capra nubiana) weighing 40-50 kg. The animals were kept at King Khalid Wildlife Research Center nearby Riyadh, Saudi Arabia. They were fed on a ration of dried lucerne and commercial concentrate (crude protein 16%), with free access to water. All females were routinely vaccinated against infectious diseases and given coccidiostats and anthelminthic drenches as necessary. females were divided into three groups, each consisting of 7 females. The first group was considered to be 30-60 days of gestation, the second 60-90 days and the third 90 forward.Blood samples (10 ml), were collected from each ibex by jugular vein puncture into clean vacuum tubes (Becton, Dickinson and Co., USA) containing EDTA-K2, while the animal was manually restrained. Each tube was inverted 2-3 times to ensure thorough mixing. The samples were analyzed within 2 h in the laboratory using an automated hematology analyzer (VetScan HM2; Abaxis Veterinary Diagnostics). Each sample was analyzed for RBC and Hemoglobin (Hb), by Counter Model ZM (Coulter Electronics Ltd., Luton, Bedford shire, United Kingdom).All samples were evaluated on the same day. Blood samples for biochemical analyses were centrifuged at 3,000 rpm for 10 min, and the serum was decanted. Then the biochemical parameters were obtained using the biochemistry analyserVetScan VS2 (Abaxis Veterinary Diagnostics, Union City, CA 94587, USA).Serum samples were analysed by automated analyserRobonik ASP-1300 for aspartate aminotransferase (AST), alanine aminotransferase (ALT), glucose, total serum protein calcium (Ca), inorganic phosphorus (P) and total serum proteins (TP).The results are obtained within 10–15 min for each rotor.
3. Statistical Analysis
- Each treatment was composed of 21 replicates (n = 21). Standard Error (SE) was calculated for each parameter. The statistical analysis was followed by LSD test.
4. Results and Discussion
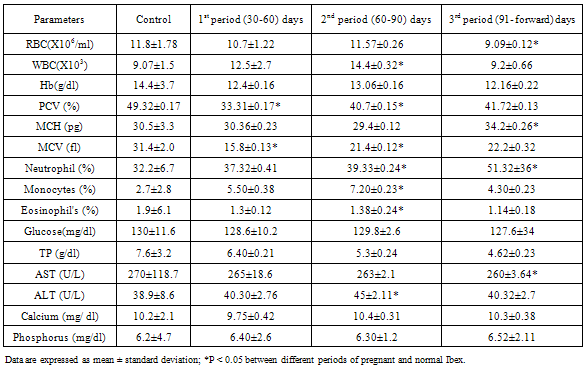
- Effects of pregnancy on the Hematological and biochemical profile of the pregnant Nubian ibexare summarized in table.1.The(RBC), (Hb), (PCV) and (MCV) were significantly decreased during pregnancy this finding is Compatibleto the study by(Calvo et al. 1989) haemoglobin decreased significantly during first half of gestation time, with the lowest values in the second period. Haemoglobin values decrease during pregnancy not only due to the utilization of the mother’s haemoglobin into foetal circulation, but also due to dilution of blood which occurs as a consequence of plasma volume increase (Roy et al., 2010) Singh e t al., (1991). (PCV) and (MCV) were also significantly decreased during pregnancy. MCV showed significant difference in the first and second period of gestation at (p<0.05).Red blood cells count (RBC), packed cell volume (PCV) and haemoglobin concentration (Hb) increased in the second period of pregnancy compared to first period and decreased in the last period for (RBC) and (Hb). A raise in (PCV) appeared in the second and third gestation period, which was significantly different only compared to the first period. (Hb) concentration was also increased in the second period and differed significantly from the third period. (MCV) was higher in third gestation period, different significantly from the control group and the second gestation period. In the first period, (MCV) was significantly lower than the other periods. (MCH) was reduced during second gestation period and being significantly different from the control group and the other pregnant groups.Fetal development that occurs in that period of pregnancy produces a greater oxygen demand. This greater need for oxygen is compensated by the endocrine system that stimulates the release of erythropoietin (Ep), which it is the primary regulator of erythropoiesis in the mammalian fetus and the adult as described by (Gordon et al., 1973), (Zanjani et al., 1974). Inthe adult, the kidney represents the major site of (Ep) production (Jacobson et al., 1957). The secretion of this circulating glycoprotein stimulates increased production of RBC in the bone marrow (Walter., 2009).
|
ACKNOWLEDGEMENTS
- The author gratefully thanks Dr. Mohammed Abdul kadersandogah veterinary from Saudi Wildlife Commission (SWC) and Mr. Welly for his assistance in technical and statistical analysis.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML