-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Zoology
2011; 1(1): 8-11
doi: 10.5923/j.zoology.20110101.02
Present Susceptibility Status of Culex Quinquefasciatus, Say to Four Insecticides at Mysore, India
Harish Kumar T. S. , Kavitha R. S. , Lahari Nag C. N., Navya G. K., R
Department of studies in Zoology, University of Mysore, Mysore, 570006, India
Correspondence to: Kavitha R. S. , Department of studies in Zoology, University of Mysore, Mysore, 570006, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
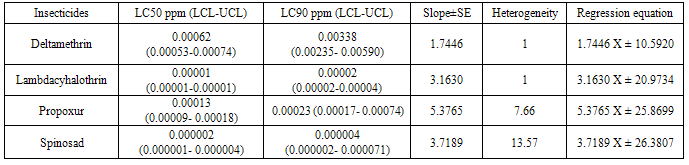
The current susceptibility status of Culex quinquefasciatus at Mysore, India to four insecticides was determined using standard WHO larval bioassay method. Culex quinquefasciatus is the primary vector of lymphatic filariasis, an endemic disease in India in 17 states and 6 union territories with about 553 million people at risk of infection. So, an effort was made to determine the susceptibility of this mosquito to 3 chemical insecticides viz Deltamethrin, Lambdacyhalothrin, Propoxur and a biocide, Spinosad. Among these Spinosad was found to be the most effective insecticide with LC50 of 0.000002 ppm followed by Lambdacyhalothrin, Propoxur and Deltamethrin with LC50 values of 0.00001, 0.00013 and 0.00062 ppm respectively. The results revealed the importance of a biocide explored from nature maybe employed to prevent pollution and health hazards.
Keywords: Culex quinquefasciatus, Bioassay, Spinosad, Biocide
Cite this paper: Harish Kumar T. S. , Kavitha R. S. , Lahari Nag C. N., Navya G. K., R , "Present Susceptibility Status of Culex Quinquefasciatus, Say to Four Insecticides at Mysore, India", Research in Zoology , Vol. 1 No. 1, 2011, pp. 8-11. doi: 10.5923/j.zoology.20110101.02.
Article Outline
1. Introduction
- Mosquitoes account for majority of the transmissions among the vector- borne diseases. They are the most important of the disease vectors that number over 350 species worldwide out of about 3500 species. Mosquito- transmitted diseases are the major cause of loss of human life worldwide, with over 700 million people suffering annually[1]. Such diseases vectored by mosquitoes in India are malaria, lymphatic filariasis, dengue, chikungunya and Japanese encephalitis. Culex quinquefasciatus is the main vector which transmits microfilariae of Wuchereria bancrofti that causes about 98% of the lymphatic filariasis cases, a major neglected tropical disease. A district-level endemicity map created for India in 2000 shows that of the 289 districts surveyed up to 1995 (62% of all districts), as many as 257 were found to be endemic. Seventeen states and six Union Territories were identified to be endemic with about 553 million people exposed to the risk of infection; and of them, about 146 million live in urban and the remaining in rural areas. About 31 million people are estimated to be the carriers of mf and over 23 million suffer from filarial disease manifestations in India[2]. Due to the absence of an effective vaccine and considerable side effects of the available chemotherapy, the main option available for controlling and preventing filariasis is the control of Culex quinquefasciatus[3]. Chemical control is one of the several methods used in integrated vector control. Since the discovery of DDT as apotent insecticide, chemical control has become the method of choice which necessitated the use of other types of insecticides as well[4]. But due to reasons such as indiscriminate use of insecticides and natural adaptation, the vector has developed resistance to many of the available chemical insecticides in many places all over the world resulting in repeated disease outbreaks. This negative impact of chemical insecticides has prompted researchers to develop new environmentally compatible vector control methods using biocides.Spinosad is a relatively new bioinsecticide, the active ingredient of which is derived from Saccharopolyspora spinosa, proving to be highly effective against mosquitoes. It kills susceptible species by causing rapid excitation of the insect nervous system. The discovery and characterization of this soil Actinomycete has presented a novel opportunity to develop a portfolio of progressive insect management tools. It has been used to control a variety of insect pests including fruitflies, sawflies, spider mites, fire ants and leaf miners[5][6]. Along with Spinosad three other chemical insecticides were also used for the present investigation in order to determine the current susceptibility status of Culex quinquefasciatus as the susceptibility status varies from region to region and from time to time. It is in this context the present bioassay was carried out in the Vector Biology Research Lab of Zoology Department at University of Mysore.
2. Materials and Methods
2.1. Collection of Mosquito Larvae
- Culex quinquefasciatus larvae were collected from breeding source of the University campus in Manasagangotri, with the help of a dipper. They were transferred to trays containing fresh water in order to get acclimatized to laboratory conditions. The late 3rd or early 4th instar larvae were separated and transferred to fresh water using Pasteur pipette just before the bioassay.
2.2. Larval Bioassay
- The standard WHO procedure was followed for determining the larval susceptibility[7]. Accordingly the larvae were subjected to different concentrations of insecticides whose stock solutions were prepared by using distilled water as the solvent. Deltamethrin stock solutions were prepared in absolute alcohol and subsequent dilutions were made as per requirements. Test concentrations were prepared by adding 1ml of insecticide containing solution to 249ml of dechlorinated water in 500ml capacity beaker. It was stirred vigorously for 30 seconds with a glass rod. For the control, 1ml of distilled water or absolute alcohol as required was added to 249ml of dechlorinated water instead of insecticide. To each of the beakers containing different test and control, 25 late 3rd or early 4th instar larvae were released with the help of a strainer. Mortality was observed after 24 hours and the data was established using Abbott’s formula[8]. All the tests were carried out at room temperature of 26±2℃ and relative humidity of 70±5%. At least two replicates were maintained for both test and control.
2.3. Data analysis
- Larval counts were adjusted for the mortality in control, if present, employing Abbot’s formula.
 The LC50 and LC90 values for each insecticide were calculated by dosage mortality regression line using Probit analysis[9].
The LC50 and LC90 values for each insecticide were calculated by dosage mortality regression line using Probit analysis[9].3. Results
- Data showing the LC50 and LC90 values along with the fiducial limits of all the insecticides employed is presented in Table 1. Deltamethrin in the concentration of 0.0002, 0.0003, 0.0005, 0.0009 and 0.0014 ppm produced an experimental mortality of 16.0%, 32.0%, 48.0%, 56.0% and 74.0% respectively with zero mortality in control. The LC50 and LC90 values were 0.00062 and 0.00338 ppm respectively. Similarly lambdacyhalothrin in the concentration of 0.000004, 0.000006, 0.000008, 0.00001 and 0.00004 ppm produced an experimental mortality of 16.0%, 30.0%, 41.3%, 62.08% and 98% respectively with zero mortality in control. The LC50 and LC90 values for this insecticide were 0.00001 and 0.00002 ppm respectively. Propoxur in the concentration of 0.00006, 0.00010, 0.00014, 0.00018 and 0.0002 ppm produced an experimental mortality of 8.0%, 16.0%, 56.0%, 72.0% and 92.0% respectively with zero mortality in control. The LC50 and LC90 values here were 0.00013 and 0.00023 ppm respectively. Similarly Spinosad in the concentration of 0.0000009, 0.000001, 0.000002, 0.000003 and 0.000004 ppm produced an experimental mortality of 8.33%, 32.0%, 40.0%, 76.0% and 100% respectively with zero mortality in control. The LC50 and LC90 values were 0.000002 and 0.000004 ppm respectively. Figure 1 depicts the percentage mortality of Cx. quinquefasciatus larvae after treating with deltamethrin, lambdacyhalothrin, propoxur and spinosad shows a linear exponential curve.
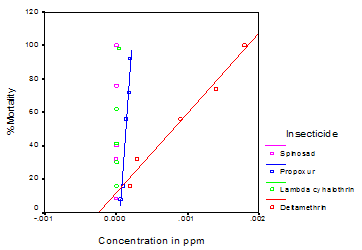 | Figure 1. Regression profile indicating % mortality of Cx. quinquefasciatus larvae after treating with Deltamethrin, Lambdacyhalothrin, Propoxur and Spinosad |
4. Discussion
- Depending on the LC50 and LC90 values of insecticides employed for the present study it can be inferred that bioinsecticide, Spinosad is highly effective as mosquito larvicide. It has the lowest LC50 and LC90 values compared to that of other insecticides which shows that Spinosad is highly effective at low concentration (Table 1).The present results are in agreement with that of Darriet et al[2005] who have observed that Spinosad had lethal action on the larvae of mosquito species Aedes aegypti, Culex quinquefasciatus and Anopheles gambiae that were susceptible or resistant to pyrethroids, carbamates and organophosphates at Montepelleir, France[10]. Liu et al[2004] have demonstrated that 3 strains of larvae of Culex quinquefasciatus which had great ability to develop resistance to different insecticides such as permethrin, deltamethrin, chlorpyrifos, fipronil and imidaclopid were highly susceptible to Bacillus thuringiensis variety israelensis and Spinosad in USA[11]. Spinosad kills susceptible species by causing rapid excitation of the insect nervous system. As spinosad is ingested by the insect, it has very little effect on non-target predatory insects. No case of bioaccumulation in water or soil has been recorded so far.The second most effective insecticide among the four was found to be Lambdacyhalothrin as per the LC50 and LC 90 values (Table 1). Though lambdacyhalothrin, a synthetic pyrethroid, is efficient as a mosquito larvicide, mosquitoes have been known to develop resistance against this pyrethroid[12][13][14]. It is extremely poisonous to aquatic organisms and when used indiscriminately it affects the aquatic ecosystem.
|
5. Conclusions
- Therefore the present investigation leads to the point that, Spinosad is a highly effective bioinsecticide against the larvae of mosquitoes and it may be valuable for the management of Culex quinquefasciatus especially in situations where local strains are highly resistant to other insecticides. Further experiments should be conducted in order to determine whether Cx. quinquefasciatus could develop resistance to this insecticide. Experiments should also be conducted regarding the susceptibility of other groups of mosquitoes against this insecticide so that the control of vectors will successfully help in the reduction of incidence of vector-borne diseases.
ACKNOWLEDGEMENTS
- The authors are extremely grateful to the Chairman, Department of studies in Zoology, University of Mysore, Manasagangothri for providing all the facilities for timely completion of this work. We also thank Mr. Vivek Charles, Ms. Prathibha KP and Mr. Puneeth P, Research Scholars, at Vector Biology Research Lab, Department of Zoology, University of Mysore, Manasagangothri for their support.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML