-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Textile Science
p-ISSN: 2325-0119 e-ISSN: 2325-0100
2021; 10(2): 21-26
doi:10.5923/j.textile.20211002.01
Received: Sep. 16, 2021; Accepted: Sep. 30, 2021; Published: Oct. 15, 2021

Livinguard Treated Textiles in the Prevention and Control of Mycobacterium tuberculosis Transmission
Shefali Mishra1, Wouter Pronk2, Mukti Mody3
1Department of Microbiology, Livinguard Technology Private Limited, Mumbai, India (VP-R&D)
2Department of Microbiology, Livinguard Technology Private Limited, Gewerbestrasse Cham, Switzerland (CTO-R&D)
3Department of Microbiology, Infexn Laboratories Private Limited Mumbai, India (HOD-Microbiology)
Correspondence to: Shefali Mishra, Department of Microbiology, Livinguard Technology Private Limited, Mumbai, India (VP-R&D).
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In this study, we examined the antimicrobial efficacy of textiles impregnated with Livinguard technology and its implications in the control of TB against Mycobacterium tuberculosis, the highly pathogenic bacteria which possess a global health challenge across the world. Polyester/cotton (65/35, 215 GSM) treated textiles with Livinguard were found to effectively kill Mycobacterium tuberculosis ATCC 25177 in 15 minutes with a very high kill rate, evident with absolutely no growth in all the MGIT liquid culture tubes of the treated textiles eluted in neutralizer. The negative result of MPT64 antigen testing further confirmed the absence of Mycobacterium tuberculosis on Livinguard textile. The use of Livinguard treated textiles in TB wards and at home can play an important role in controlling cross contamination and transmission of TB between patients, health workers and immunocompromised patients, alike.
Keywords: Mycobacteriun tuberculosis, Antimicrobial agents, Micro-Organisms, Textiles
Cite this paper: Shefali Mishra, Wouter Pronk, Mukti Mody, Livinguard Treated Textiles in the Prevention and Control of Mycobacterium tuberculosis Transmission, International Journal of Textile Science, Vol. 10 No. 2, 2021, pp. 21-26. doi: 10.5923/j.textile.20211002.01.
1. Introduction
- Mycobacterium tuberculosis is an intracellular mycobacterial pathogen that represents one of the world’s most common and most serious infectious diseases causing organism. Over 2 billion people are believed to harbour latent M. tuberculosis infections and approximately 8 million active cases of tuberculosis. Tuberculosis is the leading cause of death in the world from a bacterial infectious disease. Worldwide, TB resulted in 1.6 million deaths and was ranked as the leading cause of death due to a single infectious pathogen, even surpassing the HIV/AIDS epidemic (WHO report, 2018). Furthermore, TB, like other bacterial diseases, is confronted by problems of emerging drug resistance, which places an enormous strain on the public healthcare system (WHO report, 2018). India accounts for one fourth of the global TB burden. In 2015, an estimated 2’800’000 cases occurred and 4’800’000 people died due to TB. India has highest burden of both TB and multi-drug resistant TB based on estimates reported in Global TB Report 2016. The World Health Organization (WHO) describe it as an “epidemic” and reported that it is among the top 10 causes of death globally and “the leading cause of death from a single infectious agent.” The WHO estimate that in 2018, nearly 10 million people around the world developed TB and 1.5 million people died from the disease. Currently, antibiotic resistance is causing renewed concerns about TB among experts. Some strains of the disease are not responding to the most effective treatment options. An estimated 1’300’000 incidents of multi-drug resistant TB patients emerge annually in India which includes 79’000 MDR-TB patients estimates among notified pulmonary cases (Kwonjune et al; 2015). In this case, TB is very difficult to treat. The biggest challenge posed by the Mycobacterium is its intracellular nature which limits a variety of antibiotics ineffective for the treatment of the diseases. The cell wall complex contains peptidoglycan and complex lipids. Over 60% of the mycobacterial cell wall is lipid. The lipid fraction of MTB's cell wall consists of three major components, mycolic acids, cord factor, and wax-D. Mycolic acids are unique alpha-branched lipids found in cell walls of Mycobacterium. They make up 50% of the dry weight of the mycobacterial cell envelope. Mycolic acids are strong hydrophobic molecules that form a lipid shell around the organism and affect permeability properties at the cell surface (Kenneth Todar., 2009). Mycolic Acids are thought to be a significant determinant of virulence in MTB. Probably, they prevent attack of the mycobacteria by cationic proteins, lysozyme, and oxygen radicals in the phagocytic granule. There are very few antibiotics in the world which acts upon this organism for its treatment (eg. Rifampicin, Isoniazid etc). Secondly, the variety of its strains and serotypes posed further challenge to curb this organism and the inherent adaptability of their serotypes causes severe resistance against the antibiotics used if the dose and duration of long-term therapy is not optimized and not planned properly. Considering highly complex structure of Mycobacterial cell wall and its resistance towards various drugs, the antimicrobial fabric could be a very suitable and efficient alternative which can be used as a preventive measure for the spread of this disease and may control the development of multidrug resistant strains of Mycobacterium tuberculosis. Antimicrobial fabrics have been designed with the purpose of imparting antimicrobial activity of textiles, to protect the wearer from microbial attack, prevent the transmission and spreading of pathogenic microorganisms, inhibit odour development resulting from microbial degradation, and to create a material that will act as preventive treatment. Ideal antimicrobial finishing needs to fulfil several requirements in order to achieve the maximum benefit from antimicrobials functionalized textile products (Tijana et al., 2011). Textile together with humidity and heat, can create the right conditions for the proliferation of numerous microorganisms (Breathnach., 2013). The prevalence of microorganisms in the hospital setting could be reduced by using effective antibacterial compounds adhering to frequent contact surfaces, such as curtains, uniforms, or sheets, as well as other materials. As indicated by a number of authors, the reduction of the bacterial load on common contact surfaces should lessen the probability of a specific infectious dose of bacteria being transmitted by health workers to susceptible patients (Anderson et al., 2009).The Livinguard Technology is unlike other antimicrobial technologies in two ways; it is permanently bound to the textile with the ability to continuously destroy and/or deactivate microbes based on a physical/mechanical contact killing, and it is non-leaching. Due to these properties, the Livinguard treatment sets itself apart from other textile technologies that tend to be silver based and kill microbes by the conventional method of poisoning through slow release of the chemicals that were temporarily fixed to the textile. Due to this novel approach, many other pathogens like E.coli, P.aeruginosa, S.aureus, S.pneumoniae, K.pneumoniae, S.typhi, C.albicans, A.niger, many viruses like Influenza, Yellow fever, SARS-CoV-2, AMR (Anti-microbial Resistance) bacteria such as MRSA and VRE have been effectively killed through the use of Livinguard antimicrobial technology. In consideration of the above facts, this study was conducted to examine the survival of Mycobacterium tuberculosis pathogen on Livinguard treated fabric and to evaluate its efficacy in vitro condition.
2. Material and Methods
- Bacterial strainStock culture of Mycobacterium tuberculosis ATCC 25177 was streaked on Lowenstein Jensen slant medium and incubated at 37 °C ± 1 °C for 7-10 days. Further, from this inoculum, bacteria were scrapped out from the breeding surface of the slant and transferred to MGIT liquid culture tube. The final concentration was maintained 1.2 x 109 cfu/ml in MGIT liquid culture using McFarland standards. This was used for test fabric surface inoculation.Test materials and biocidal efficacy testingThe test fabric (Livinguard CWS recipe treated) polyester/cotton (65/35, 215 GSM) was used for the experiment. Untreated polyester/cotton (65/35, 215 GSM) fabric was used as a control (CF). Autoclaved four to five swatches of 48 mm discs of control and treated fabrics were placed in Petri dishes separately and 1000 μL of a bacterial cell suspension were smoothly deposited on their surface. Petri dishes were maintained at a room temperature of 37°C and between 40-60% of humidity for 15, 30, and 60 minutes. Fabrics were then removed using sterile forceps and introduced separately in a sterile 250 ml Erlenmeyer flasks containing 100 ml of DEN broth. The flasks were vortexed for 30- 60 seconds and shaken vigorously by hand for 1-2 minutes for extraction. MGIT liquid culture for the growth of Mycobacteria0.5 ml of neutralized solution from each contact time (15 mins, 30 mins and 60 mins) were immediately inoculated to 8 ml of readymade MGIT (Mycobacteria growth indicator tube) liquid broth and incubated @ 37°C for six weeks. All tubes were kept inside BACTEC MGIT 960 system for incubation.The instrument signals a tube positive for tubes where growth has occurred (based on fluorescence), and an indicator green light shows the exact location of the positive tube in the drawer of the instrument. At this point, the tube should be removed and scanned outside the instrument. The tube should be observed visually. Mycobacterial growth appears granular and not very turbid while contaminating bacterial growth appears very turbid. Growth, especially of the M. tuberculosis complex, settles at the bottom of the tube. The incubation time for which growth was observed must be recorded. ZN stain of the Positive MGIT tubesOnce a MGIT tube was positive by fluorescence or by visual observation, prepared a smear and stained with ZN stain. Used a clean slide. Mixed the broth by vortex and then by using a sterile pipette, removed and aliquot 1mL of broth in sterile falcon tube. Placed 1-2 drops on slide and spreaded it over. Kept the smear for air drying. Heat-fixed the smear by passing it over a flame for few times and stained the smear with Ziehl-Neelsen stain and kept for air drying. Observed under microscope. Screened under a low power objective (10x) to locate stained bacteria and then switched to an oil immersion objective (100x) lens for detailed observation. MPT64 antigen testing All smear positive for pure AFB (irrespective of presence or absence of a typical granular growth in the MGIT media indicating growth of MTB) must be proceeded with MPT64 antigen testing for the confirmation of a Mycobacterium tuberculosis. If the sample is positive for MPT64 report, it gives the 100% confirmation for the presence of Mycobacterium tuberculosis. Once the instrument (BACTEC MGIT 960) flags result as negative, removed the tube from the instrument by scanning and prepare a smear and stain with Z N stain. MGIT flag negative and smear negative for AFBs can safely be reported as negative for Mycobacterium species.
3. Results
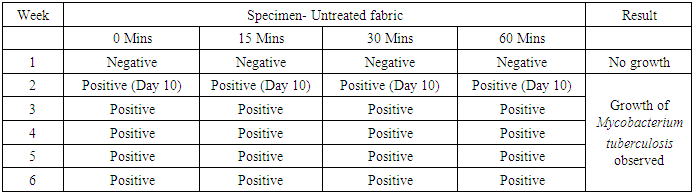
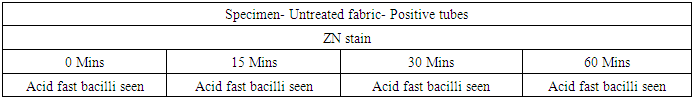
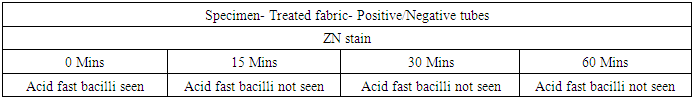
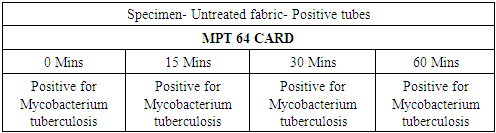
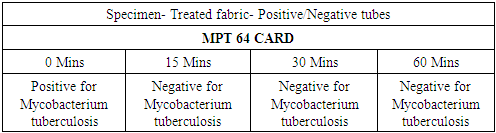
- In the first part of this study, all untreated fabric samples from four contact times were found positive for Mycobacterium tuberculosis in MGIT liquid culture, whereas all treated fabric samples found negative for MTB growth in 15 mins, 30 mins and 60 mins of contact time. Zero minute contact showed positive for Mycobacterium tuberculosis both in case of treated and untreated fabric. MGIT liquid culture tubes were positive due to presence of live Mycobacterium tuberculosis cells which got killed in 15 minutes of contact. No growth observed in first week of incubation, and it appeared in all positive tubes after 10 days of incubation period (Table 1a and Table 1b, Fig. 1).
|
|
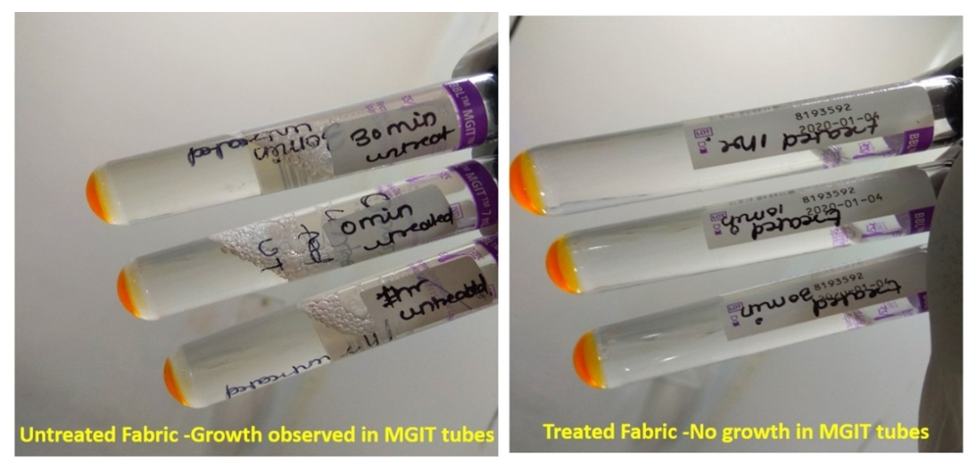 | Figure 1. Growth in MGIT tubes |
|
|
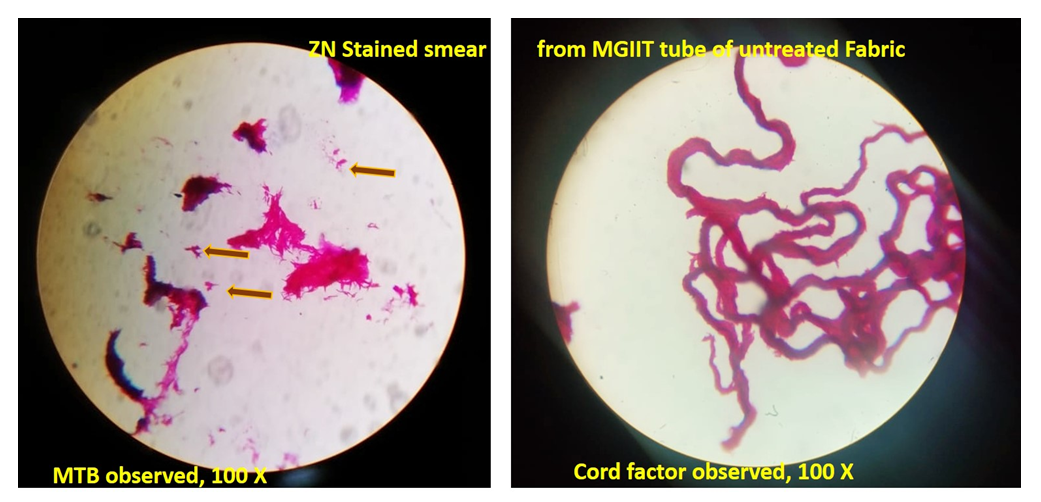 | Figure 2. Microscopic observation of ZN stained slides (Untreated fabric) |
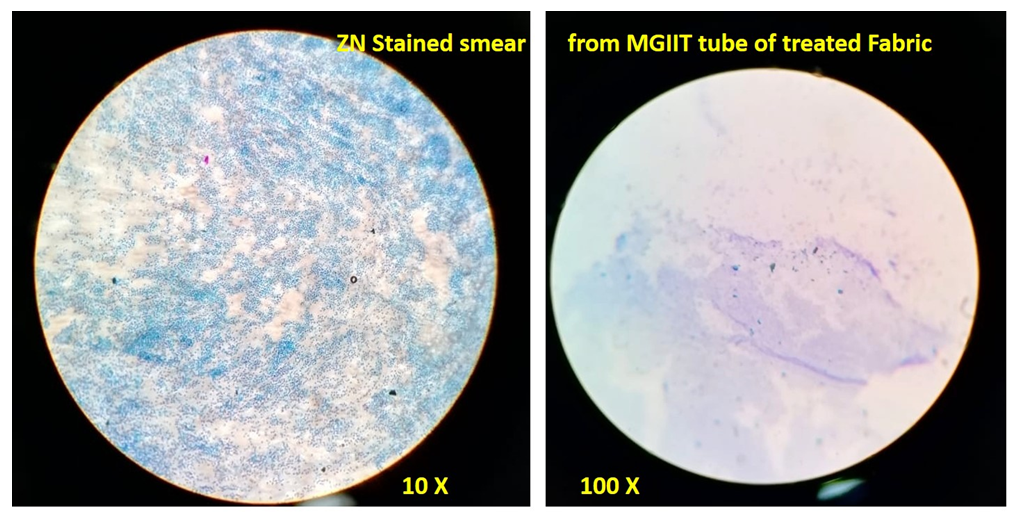 | Figure 3. Microscopic observation of ZN stained slides (Treated fabric) |
|
|
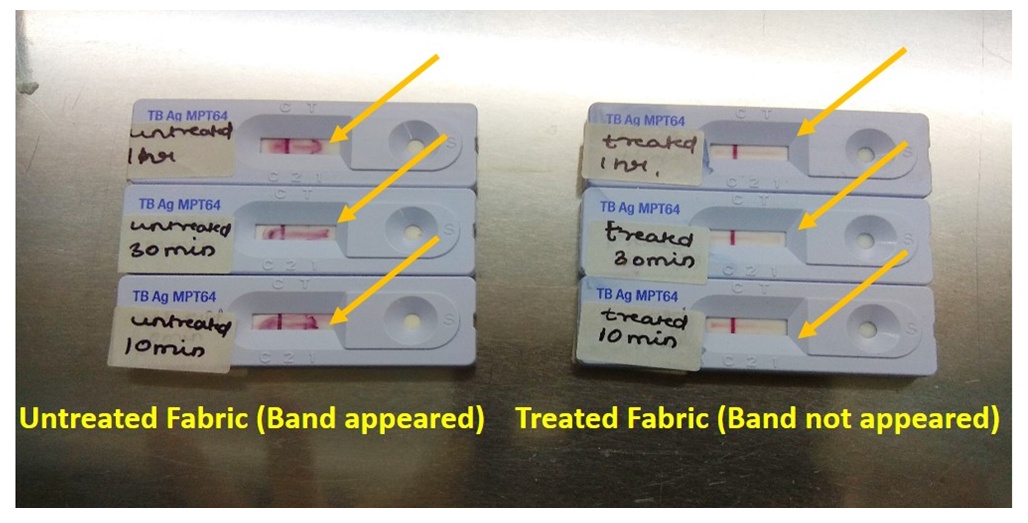 | Figure 4. MPT 64 CARD test |
4. Conclusions
- Nosocomial infections become a major cause of morbidity in patients of all age groups due to the increasing capacity of bacterial pathogens to acquire resistance to classical anti–infectious agents (Shanmugasundaram et al., 2013). Over a period, many strategies have been developed to reduce nosocomial infection rate, despite that the infection rate remain high, thus it needs an efficient technology to counter the problem holistically and to curb the rate of infection at the source of entry of the pathogen itself. There is an urgent need to develop functional fabrics having antibacterial properties for the use on the surfaces which comes in frequent contact with the patients, such as uniforms, bedding, curtains, and upholstery (Borkow and Gabbay, 2008; O’Hanlon and Enright, 2009). Several methods have been used to determine the efficacy of Livinguard antimicrobial textiles. Among all the methods, the MGIT liquid culture and MPT64 CARD tests found to be the most reliable one. All fabric surfaces were inoculated as per AATCC-100 (2012) standard where the bacterial inoculum was deposited on the test fabric surface and kept inside the incubator at 37°C for different contact intervals. Afterwards, the absorbed bacteria were eluted and transferred to MGIT liquid culture tube for further analysis.The study revealed that the Livinguard technology can effectively kill Mycobacterium tuberculosis ATCC 25177 in a very short span of time with a very high efficiency, evident with absolutely no growth in all the liquid samples of the treated textile however, the non-treated textile sample showed positive growth in all the MGIT liquid culture tubes. Although it is not easy to have the sterility of hospital premises, and hygiene and sanitation is always a challenge to maintain at such places, thus the best possible solution to avoid the nosocomial infection spread is to provide the Livinguard technology enabled PPE’s which can effectively kills the cause of the infection at source itself and protect the community who are at the higher risk of occupational hazard (eg. healthcare workers, doctors, patients, attendants etc). With the help of Livinguard technology enabled PPE like treated gowns, drapes, face masks, gloves, wipes, scrub suits etc. can ensure the absolute safety and killing of Mycobacterium tuberculosis therefore can be used effectively in the prevention of TB infection and controlling the MTB spread from one person to another, as the organism is horizontally transmitted and contagious in nature. Livinguard technology can create a disinfecting barrier in the form of a face mask or any form of textile applications in hospitals, homes and other public places and could prevent the person from occupational hazard also. The added advantage of this technology is that the reusable materials composed of cotton/polyester blends causes lesser solid waste from limited disposal and more comfort to the wearer because of their better water vapour transmission ability compared to nonwoven material.The role of textiles in infection transmission has been documented extensively, but the role of treated textiles in controlling infections without contributing to antimicrobial resistance is pioneering. This study proves the efficacy of Livinguard treated fabrics on a highly pathogenic microbe in a very short span of time. Supported by clinical studies, the use of Livinguard treated textiles in TB wards and at home can play a game-changer in the fight against TB. By controlling cross contamination and transmission of TB among patients, health workers and immunocompromised patients, alike, the public health community can effectively manage patients living with TB and significantly work towards reducing the burden of disease globally.
ACKNOWLEDGEMENTS
- Authors wish to thank and acknowledge the support given by Infexn Laboratories Private Limited (Thane, Mumbai). Infexn Laboratories is actively involved in various National disease control programs and is an approved Laboratory under the “Culture-DST Scheme for the diagnosis and follow up of Multi Drug Resistant TB (MDR-TB). We would like to thank Dr. Sonal Rajesh Bangde for her helpful suggestions and contribution in the research work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML