-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Textile Science
p-ISSN: 2325-0119 e-ISSN: 2325-0100
2019; 8(1): 10-15
doi:10.5923/j.textile.20190801.02

Surface Modification of Cotton Fabric with Effect Pigment - A Review of Improved Textile Optical Functionalization
Mohammad Mamunur Rashid1, Boris Mahltig2, Khairuzzaman Mamun3
1Master in Science, Department of Textile and Clothing Technology, Niederrhein University of Applied Sciences, Mönchengladbach, North Rhine-Westfalia, Germany
2Faculty of Textile and Clothing Technology, Niederrhein University of Applied Sciences, Mönchengladbach, North Rhine-Westfalia Germany
3Khairuzzaman Mamun, Mechanical Engineering Department, Iwate University, Iwate, Japan
Correspondence to: Mohammad Mamunur Rashid, Master in Science, Department of Textile and Clothing Technology, Niederrhein University of Applied Sciences, Mönchengladbach, North Rhine-Westfalia, Germany.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The review will introduce the surface modification of cotton textile with effect pigments to improve the optical functionalization of textiles. In this review, short description of two major groups of effect pigments- metallic effect pigment and pearl luster pigment will be discussed focusing on their optical characteristics while coated on textile. Orientation of pigment particles on coated textile, pigment-fabric bonding mechanism on coated textile and finally SEM images will be analyzed to understand the surface of modified textile in micro-scale observation. Hence, the possibility of improving optical functionalization of textile by treating with effect pigment shall be discussed in this work.
Keywords: Surface modification, Effect Pigments, Textile Coating, Textile Functionalisation
Cite this paper: Mohammad Mamunur Rashid, Boris Mahltig, Khairuzzaman Mamun, Surface Modification of Cotton Fabric with Effect Pigment - A Review of Improved Textile Optical Functionalization, International Journal of Textile Science, Vol. 8 No. 1, 2019, pp. 10-15. doi: 10.5923/j.textile.20190801.02.
Article Outline
1. Introduction
1.1. Effect Pigment
- Effect pigments consist of flakes or lamellae of aluminum (aluminum bronzes), copper and copper–zinc alloys (“gold bronzes”), zinc, and other metals [1]. Metallic effect pigment and pearl luster effect pigment are the two types of effect pigments. The metallic effect is caused by the reflection of light at the surface of the pigment particles [2]. Effect pigments cannot be applied alone onto textile surface, because the adhesion of pigment to the textiles surface is not sufficiently high. A binder is necessary to fix the pigment onto the textile properly. Sometimes a dispersing agent is added to disperse the other chemical in the solution. Through this combined application of binder and pigment as one coating system, several new parameters influence the final coating [3]. Pigments are classified according to their interaction towards light such as white pigment, color pigment, black pigment and effect pigment. [4]. Effect pigments are two types; metal effect pigment and pearl luster pigments. Effect pigments give additional color effects when they are applied on fabric. Metal effect pigments are not transparent and therefore reflects all the incident light in one direction. On the other hand, pearl luster pigmets reflect part of incident light, allowing a part of incident light enter into the transparent and partially reflect either at interface or at the bottom of platelets. Effect pigments are originally used to get special optical effects of color and light reflectivity [3]. Most important class of effect pigment now-a-days is mica coated with different metal oxide such as iron(III) oxide, tin dioxide, chromium (III) oxide [2,4]. The influence of pigment surface composition, embedding into the coating or scattering effects, is discussed as having an influence on the optical properties and effects of those special pigments [5].Different effect pigments contain different nanoparticles like TiO2, ZnO, Al2O3, Ag content etc. A sol–gel based surface treatment can be carried out for different purposes like preparation of water repellent antistatic textiles [6, 7], for antimicrobial effect [8], preparation of metallized titania sols for photocatalytic coating [9]. Improved stability against mechanical or thermal destruction, improved water, oil and soil repellency, improved electric conductivity, changing of light absorption of textiles, at small film thickness, sol–gel coatings feature excellent scratch, scrub and weathering resistance along with superior thermal stability deodorizing and UV absorbing material can be obtained with ZnO coated fabric were found through different researches [10-12]. Photocatalytic titania coating can be done by sol-gel process [13]. Zinc oxide nanoparticles can be prepared by the sol–gel method which is more popular because of its cheapness, reliability, repeatability, and simplicity and show good optical properties [14]. Titanium dioxide nanoparticles have been used to achieve antibacterial/antimicrobial properties [15-19]. Different researchers showed its self-cleaning property while applied on textile [20-23]. TiO2 is also used for its air purification, photocatalytic [24-26] and UV-protection characteristics [27, 29]. Surface modification by TiO2 also carried out for obtaining hydrophilic or ultra- hydrophobic properties [30]. Radetić discussed functionalization of TiO2 nanoparticles treated textile materials focusing on Self-cleaning, UV protection, antibacterial and photocatalytic activity [31]. The performance of ZnO nanoparticles as UV absorbers can be efficiently transferred to fabric materials through the application of ZnO nanoparticles on the surface of cotton. [32-36].
1.2. Metal Effect Pigment-Binder System
- The use of pigment powders has the advantage that higher pigment concentrations which can be easily realized in the final coating. In the application process of coating, effect pigments are applied onto textile surfaces with binder in order to increase the adhesion of pigment. It helps pigment to be penetrated properly to textile. Due to combined application of binder and pigment as one coating system, several new parameters influencing the final coating properties have to be taken into account [3]. During the drying process after application of the pigment paste onto the substrate, a kind of self- orientation can lead to a horizontal orientation of the effect pigments inside the coating [37].
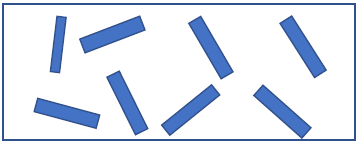 | Figure 1. Random orientation of pigment |
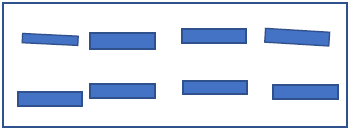 | Figure 2. Parallel orientation of pigment after drying and condensation |
2. Reflection Mechanism
2.1. Reflection Mechanism of Metal Effect Pigment
- The most important effect pigments without a layer structure are by far the metal effect pigments. Metal effect pigments consist of small metal platelets (for example aluminum, titanium, copper) which operate like little mirrors and almost completely reflect the incident light. The metallic effect is caused by the reflection of light at the surface of the pigment particles. The so observed luster effect is decreased when the part of the light scattered at edges and corners of the particles increases. Larger particles are better reflectors leading to higher brilliance and brightness. The metallic appearance depends also on the orientation of the metal flakes in the application system, the particle shape, the transparency of the binder matrix, and the presence of other colorants.
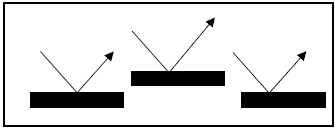 | Figure 3. Light reflection by metal effect pigment particle [4] |
2.2. Reflection Mechanism of Pearl Luster Pigment
- Pearl luster pigments simulate the luster of natural pearls. These consist of alternating transparent layers with differing refractive indices. The layers consist of CaCO3 (high refractive index) and proteins (low refractive index). This difference in refractive indices, arising equally on the interface between air/oil film or oil film/water, is a prerequisite for the well-known iridescent color images in these media. Small highly refractive platelets of pearlescent pigments align themselves parallel in optically thin systems such as paints, printing inks, or plastics. Interference effects develop when the distances of the various layers or the thicknesses of the platelets have the right values.
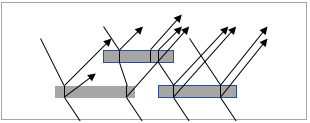 | Figure 4. Light reflection by pearl luster pigment [4] |
3. Structure of Coated Fabric
- A three dimentional structructure is produce, when metal oxide sol-gel binder system is applied on textile substrate. Because of large surface to volume ratio, it creates a solvent-containing lyogel layer on the textile surface. A xerogel layer is obtained from lyogel by drying and curing to remove solvent from the coating. So, this process produce an oxide layers on textiles. These oxide layers are stable against light, heat, chemical and microb. [38]. The hydrolysis and drying conditions govern the density, porosity, critical thickness for cracking and the mechanical properties of the layers. Mahltig et al. discussed the combination model of jing et al. [2007] and kozuka et al. [kozuka 2000].
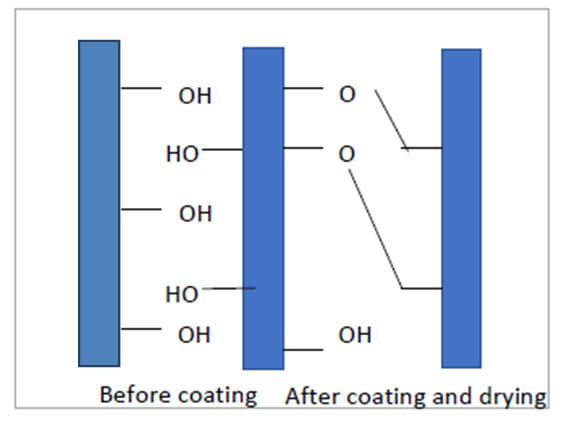 | Figure 5. Coated fabric structure followed by mahltig et al. [2008] |
4. Developed Optical Functionalization Observed by SEM Images
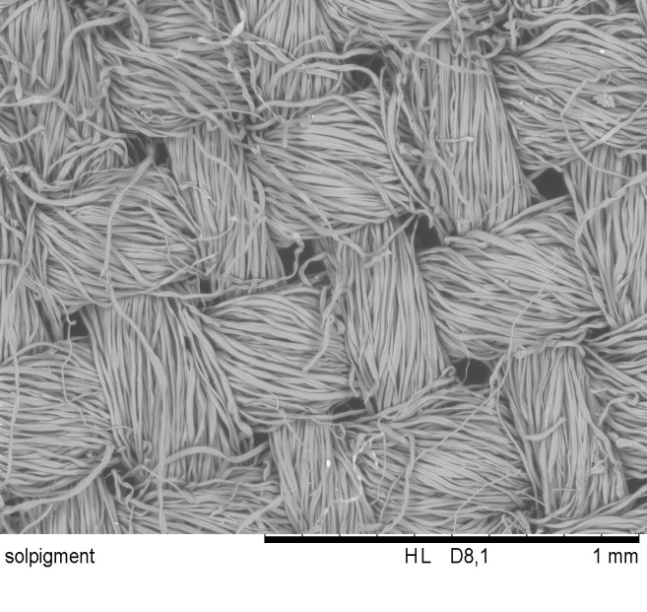 | Figure 6.1. Reference cotton (x100 times magnification) |
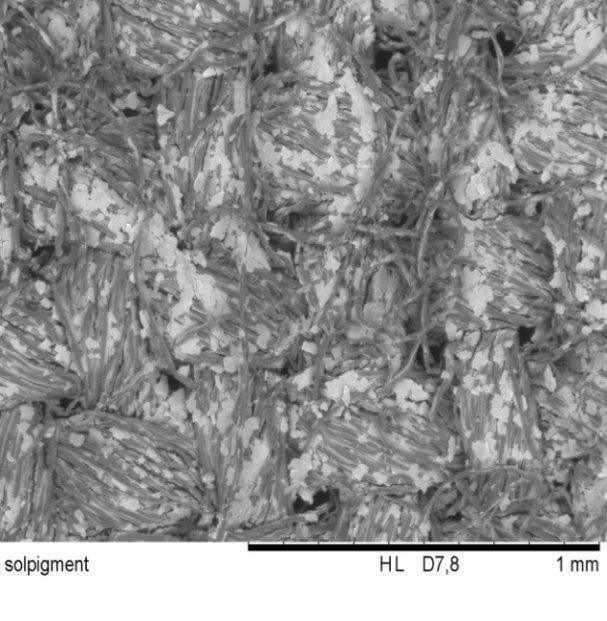 | Figure 6.2. Symic C001 coated textile (x100 times magnification) |
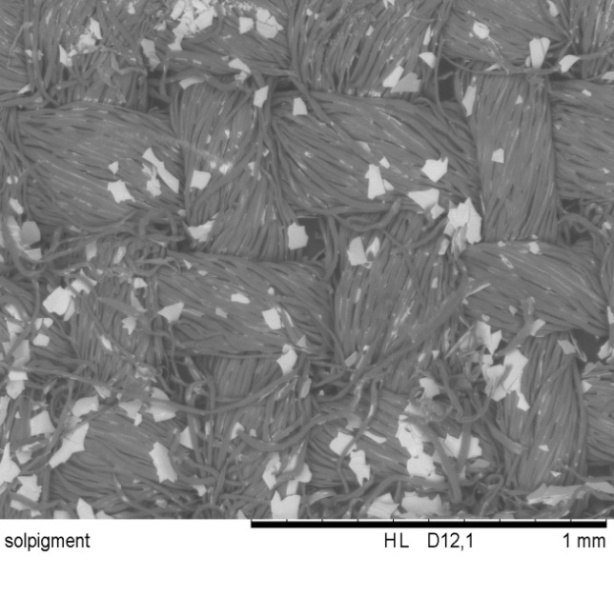 | Figure 6.3. Luxan D393 coated textile(x100 times magnification) |
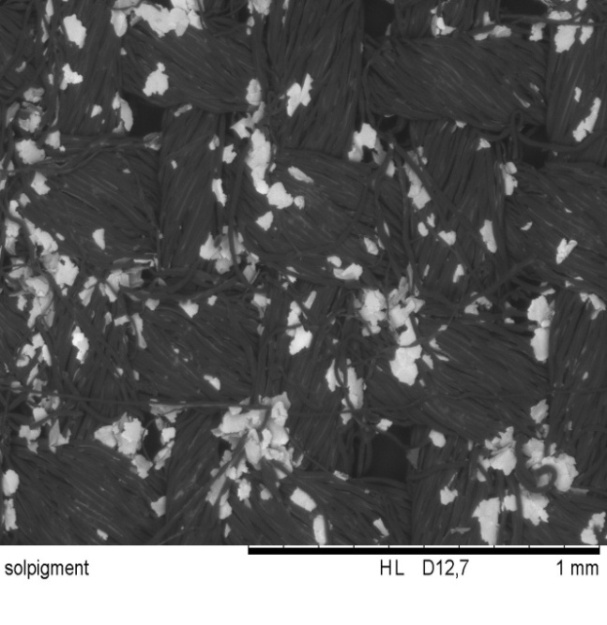 | Figure 6.4. Conduct 421000 coated textile (x100 times magnification) |
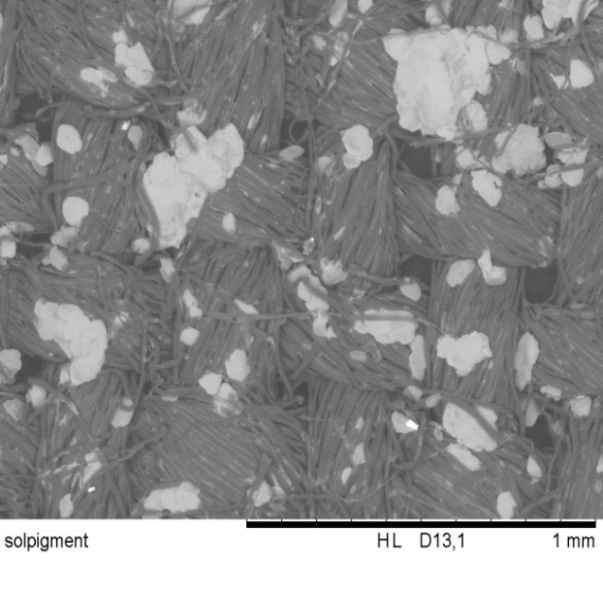 | Figure 6.5. Texmet 5000 coated textile(x100 times magnification) |
5. Textile Materials and Radiation Protection
- The light we get from sun is although essential for living organism, a part of this is absorbed when it penetrates the sample, and is converted into a different energy form. The portion of radiation that travels through the fabric and reaches the skin which is called transmission component. This part is harmful for the skin. Ultraviolet rays constitute a very low fraction in the solar spectrum but influence all living organisms and their metabolisms. The relationship between skin cancer and UV dosage is well correlated. Research showed that excessive UV radiation leads to cell damage and causes inflammation of human skin such as UV rays, dosing at 15 – 30 mJ/cm2, burn white skin easily after 5-10 minutes, has the highest risk of premature skin ageing and greatest risk of developing skin cancer [38]. Insufficient protection by clothing might contribute to the skin cancer epidemic and to the induction of epithelial skin cancers and create health problems in patients suffering from photosensitive disorders such as lupus erythematosus, photo-allergies or chronic actinic dermatitis [39]. Another alarming sign is the destruction of ozone layer. The ozone layer has been decreased about 5% in the past 13 years at 490S. So it is high time for thinking UV protection considering increased ozone hole especially in southern part of globe [40]. Chemical modification is realisable by co-condensation with additives, which are able as co-reactants to form covalent bonds to the metal oxide matrix. Treating textile substrates with a solution containing metal oxide nanosols, the nanoparticles condensate and aggregate into a three-dimensional network called lyogel layer on the textile surface. After dried and heated xerogel layer is obtained. This structure of coating provides fabric protection these oxide layers are stable against light, heat, chemical and microbial attacks [41]. Using of metallic coating on textile, the transmission of its reference cotton fabric can be changed.
6. Conclusions
- Although effect pigments are mainly used for obtaining various color effects on applied surfaces. The use of it on textile surface enhance the optical functionalization of coated textile due to good bonding between textile and pigment nano-particle. Developed light reflection of textile makes the surface modification of textile using effect pigment great approach in near future in the field of pigment coating technology.
ACKNOWLEDGEMENTS
- We would like to thank Thomas Heistermann, Hochschule Niederrhein, for supplying the chemicals as well as technical help.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML