Kawser Parveen Chowdhury
Department of Wet Process Engineering, Faculty of Textile Chemical Engineering, Bangladesh University of Textiles, Dhaka, Bangladesh
Correspondence to: Kawser Parveen Chowdhury, Department of Wet Process Engineering, Faculty of Textile Chemical Engineering, Bangladesh University of Textiles, Dhaka, Bangladesh.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
In this study, both woven and knit fabrics were taken to evaluate the performance of water repellent finishes on cotton fabrics properties. Here, 100% cotton fabrics were treated with different types of fluorocarbon based water repellent chemicals at different formulations. The levels of water repellency of the fabrics were measured in accordance with AATCC 127 hydrostatic head test method and with ISO 4920:2012 spray rating test method. To assess the performance of water repellent finishes on fabric properties, GSM, bursting strength, tensile strength, abrasion resistance, air permeability, color fastness to wash, water, perspiration and rubbing fastness with ISO method were done. The results showed that the water repellent finish type, concentration and curing temperature were very important parameters to obtain water repellent fabrics with desirable properties.
Keywords:
Water repellency, Water repellent finish, Knit fabric, Woven fabric, Fluorocarbons
Cite this paper: Kawser Parveen Chowdhury, Performance Evaluation of Water Repellent Finishes on Cotton Fabrics, International Journal of Textile Science, Vol. 7 No. 2, 2018, pp. 48-64. doi: 10.5923/j.textile.20180702.03.
1. Introduction
Cotton is a versatile fibre with outstanding quality regards comfort ability. Water repellency is one of the most common functional properties that is needed for protective clothing without affecting the comfort ability. Water repellency is defined as the ability of a textile material to resist wetting. The tendency of a water droplet to spread out over the fabric surface mainly depends on the contact angle of the water droplet and the fabric surface. Water repellent textiles have many uses including industrial, consumer and apparel purpose. This repellency can be achieved by implementing a thin surface layer of water repellent chemicals on textile fibres. Water repellency can be done by the modification of surface energy of textiles with minimal effects on other functional properties like strength, flexibility, breathability, softness etc. [1, 2] Polymeric coating on cotton fabric must secure good homogeneity with preferred properties without deteriorating fabric’s comfort ability like handle, breathability etc. When a water repellent chemical is applied on cotton fibre, a monomer that was present in water repellent chemical with the help of initiator mixture are absorbed onto the fibres and made the fabric water repellent by formation of the polymeric chains and graft bonds inside the textile structure. Furthermore, textile materials with modified surface can be obtained even by using low add-on of the monomer by the interpenetration of components and homogenous distribution of that monomers into the fibre. There has been a market increase in the commercial use of fluorochemicals in recent years, particularly to impart water repellency to cotton. It has been reported that various types of fluorochemicals that are used for textile finishing, mainly used to impart water repellency along with oil repellency. [3-8] Because of this wide and growing use of fluorochemicals, there are some different and sometimes conflicting views as to the most efficient and effective product among all fluorochemical based water repellent chemicals. It consists of perfluorinated carbon chains incorporating a polymer backbone with perfluoro groups as its side chains. [9] Some existing fluorochemicals are made with C8 carbon backbone chains that can releaseper fluorooctanesulfonate (PFOS) and perfluorooctanoic acid (PFOA) and other toxic and hazardous materials. Hence C6 based fluorocarbons were introduced, though their repellency performance and durability are less than C8 based ones. [10] Fluorochemical finishing chemicals are usually available as water emulsions and are used to fabric by the pad-dry-cure method with a curing temperature around 10-170°C for a couple of minutes. [4-7] The water repellent propertieswere evaluated by measuring contact angle, wettability, moisture absorption and vapour permeability. The first group of water repellent finishing agents is dispersion of fluorine compounds, namely fluorocarbon. The final polymer, when applied to a fibre, should form a structure that presents a dense CF3 outer surface for maximum repellency. A typical structure is shown in below Fig 1. The length of the perfluorinated side chains should be about 8 carbons. Co-monomers are X, Y, for example are stearyl- or lauryl-meth-acrylate, butyl-acrylate, methylol- or epoxy-functional acrylates. 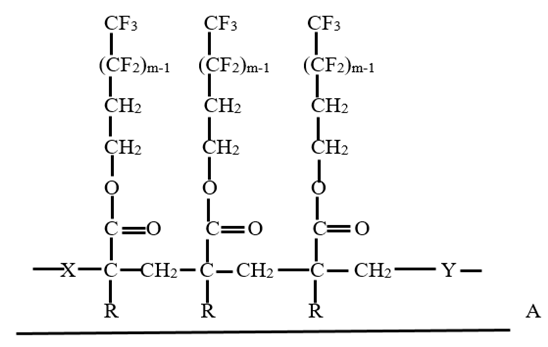 | Figure 1. Fluorocarbon repellent on fibre surface. M=8. X and Y are co-monomers, mainly stearylates. R=H or CH3 (polyacrylic or polymethacrylic acid esters). A is the fibre surface. [11] |
The second group water repellent chemical is fluorocarbon resin with polymeric, hyperbranched dendrimers in a hydrocarbon. It is a novel FC development, is inspired by nature and therefore called bionic finishes. Fluorocarbon polymers are applied together with dendrimers, where the fluorocarbon chains are enriched on the surface and crystallize with the dendrimers. Dendrimers are highly branched oligomers with non-polar chains forming a starbrush structure. They force the polar parts of the FC polymers to form the surface structure. The resulting polar and non-polar sandwich arrangements are highly ordered, causing better repellency effects, with lower amounts of fluorocarbon compared to dendrimer-free FC finishes. 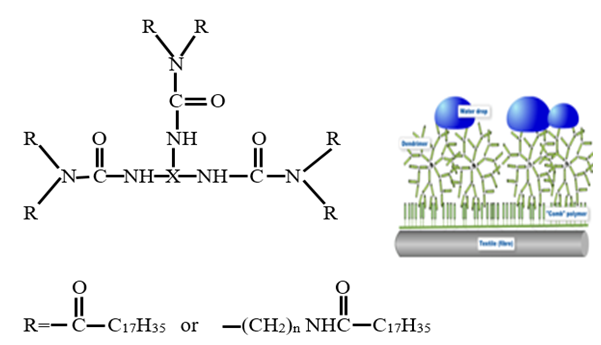 | Figure 2. A dendrimer structure synthesized from three distearyl-amines or amides and a trifunctional isocyanate X(N=C=O) [11] |
Third group water repellent chemical structure is same as first one but fluorocarbon withisocyanate booster and the length of the perfluorinated side chains should be about 6 carbons. [11]In the literature, a considerable number of studies on different water repellent chemicals, their application procedure, methods for upgrading the durability and their wash fastness and curing temperature have been reported. [12-15] There are almost insufficient work on the performance of fluouocarbon based water repellent finish and their effects on 100% cotton fabrics properties. The purpose of this study was to observe and evaluate the performance of what water repellent finish and on which concentration are the most effective on both woven and knit fabric properties after finishing.
2. Materials and Methods
2.1. Fabrics
100% cotton one woven and one knit fabrics (scoured and bleached) were used for this experiment to apply different types of water repellent chemicals by pad-dry-cure method. The woven fabric was collected from local shops and knit fabric is provided by Essential Clothing Ltd, gazipur, Bangladesh. Knit fabric had single jersey (S/J) structure with 150 GSM and woven fabric had plain weave structure with 110 GSM. At first the fabrics were dyed by using following recipe in Butex lab.Dye application bathRecipe:Dyestuffs:NOVACRON® Yellow FN-2R: 0.20%NOVACRON® Brilliant Red FN-3GL: 2.00%NOVACRON® Blue FN-R: 0.20%Auxiliaries:ALBAFLOW® FFA-01 (Penetration accelerant): 0.3g/lALBATEX® DBC (Protective Colloid): 1.0g/lBasic Chemicals:Glauber’s salt: 50g/lSoda ash: 18g/lTemperature: 60°CTime: 30 minM:L: 1:8After treatment:Hot wash at 90°C for 10 minute and then dry.These fabrics presents a 0° contact angle with water, as the drops were absorbed immediately showing excellent hydrophilic character of cotton. Supplied fabrics were first dyed and then processed with three different water repellent chemicals in three different concentrations with three different curing temperatures to investigate the effect of water repellent chemicals, their concentrations and curing temperature on water repellency and it’s durability after washing and other physical properties of the fabrics.
2.2. Water Repellent Chemicals
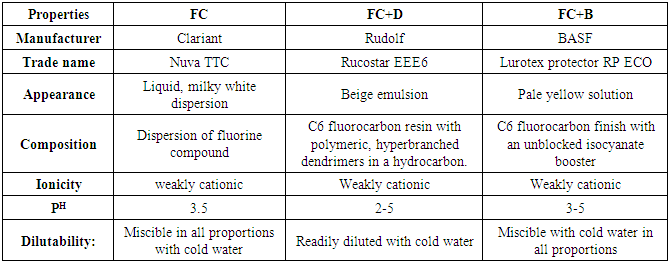
Three water repellent chemicals were used in this experiment. First water repellent chemical is fluorocarbon (FC), second is C6 fluorocarbon with dendrimers (FC+D) and third is C6 fluorocarbon withisocyanate booster(FC+B) and these water repellent chemical’s trade names, types and properties of all the commercial grades for finishing are listed in Tab. 1. and found from respective chemical’s brochure. Table 1. Trade names and types of all water repellent chemicals are listed
 |
| |
|
2.3. Water Repellent Application Bath
Woven and knit fabrics were treated with three different water repellent chemicals at three different concentrations (10 g/L, 30 g/L and 50 g/L) from a separate bath with the similar bath condition. Fluorocarbon (FC), fluorocarbon with dendrimers (FC+D) and C6 fluorocarbon with isocyanate booster (FC+B) water repellent chemical’s solutions were prepared. The process parameters were adopted as recommended by the supplier. The name of chemicals, trade name and bath set up are given in Tab. 2. Pad-Mangle machine was used for padding with 2.5 m/min fabric speed and 2.8 kg/cm2 padding pressure. Fine oven machine was used for drying and SDL Mini-Dryer Steamer was used for curing.Table 2. Water repellent application bath
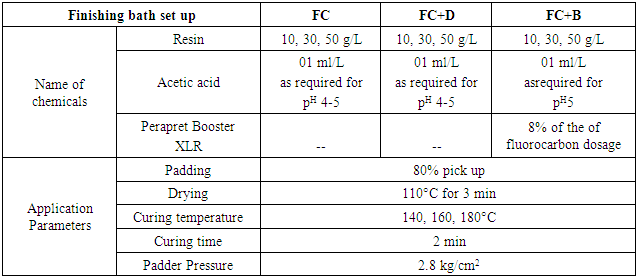 |
| |
|
2.4. Water Repellent Evaluation Test
Each samples were tested in the standard atmosphere, 25±2°C temperature and 65% RH after conditioning 24 hrs. There are three types of water repellent evaluation tests were used. 1) Absorbency test2) Drop test (it checks the contact angle)3) Spray rating test (AATCC 22-2001 test method is used to evaluate the water repellency of the fabric. Spray rating tests were done by Spray Rating Tester by James H. Heal & Co. Ltd. Halifax, England).Table 3. AATCC Spray Rating
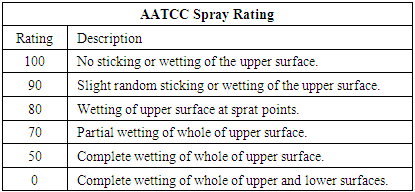 |
| |
|
4) Hydrostatic Head Test (it means water permeability test by which how much the pressure is required to penetrate the water into the fabric is measured. After water repellent finish, it is necessary to know how much the pressure increased to force the water through a fabric. It was done by Shirley hydrostatic head tester, England according to AATCC 127 method.
2.5. Physical and Chemical Testing
1) GSM test: It was done by GSM cutter from James H. Heal & Co. Ltd. Halifax, England according to ASTM (D 3776-79) method.2) Air permeability test: It was done by Air permeability tester by using ISO 9237 method3) Tensile strength test: It was done for woven fabric according to ASTM D 5045 method by Good brand fabric strength tester4) Bursting strength test: It was done by Trust brust tester according to ASTM (D 3786-87) method.5) Abrasion resistance: The abrasion resistance was determined with the weight decreasing rate and it was done by Martindale abrasion tester following ASTM D4966 method. 6) Color fastness to wash: Color fastness to wash was measured with ISO 105 C03 method. 7) Color fastness to water: It was done by ISO 105 E01 method.8) Color fastness to perspiration: The resistance of color against acid and alkali of dyed fabrics were done by ISO 105-E04 method. 9) Rubbing fastness: The resistance of color against rubbing of dyed fabrics (dry and wet) were evaluated with ISO-105-X 12 method.
3. Result and Discussion
Three individual fluorocarbon based water repellent resins such as fluorocarbon (FC), FC+dendrimers (FC+D) and FC+booster (FC+B) were chosen in this work and to evaluate the effect of different concentrations of that resins’ solutions on water repellency, solution’s concentrations were varied to 10 g/L, 30 g/L and 50 g/L and were applied on plain weave woven and single jersey (S/J) knit dyed fabrics. Meanwhile, the effects of different curing temperatures were also determined on water repellency by varying curing temperatures (140°C, 160°C and 180°C). The durability of all the fluorocarbon finished cotton fabrics (woven and knit) were investigated after repeated laundering in terms of water repellency.
3.1. Water Repellency Evaluation Test
1) Absorbency test (Spot test)In a pipette a solution of 1% direct red (Congo red) is taken and droplet of solution put on the different places of the fabric. The shape of the absorption area on the fabric is observed from below Fig 3. (a) Only scoured and bleached woven white fabric sample, two droplets are seen with 1.5 cm dia. as no water repellent chemical were present and for this reason, the drop get absorbed and spread on the surface of the fabric; (b) For woven fabric dyed and water repellent finished, the water droplets do no spread or get absorbed by the surface and so, in photography, the water drop can’t be seen; (c) For only dyed S/J knit fabric, droplet was absorbed and spread with diameter of 1.8 cm as the fabric was only dyed without finishing, so the liquid easily penetrated through the fabric; (d) dyed and finished S/J fabric, the liquid could not penetrate through the fabric surface and that’s why the liquid drop could not be seen on fabric, (e) Finished S/J white fabric after the absorbency test, the liquid could not penetrate through the fabric surface and for that reason the liquid drop could not be seen. | Figure 3. (a) For only scoured and bleached white woven fabric sample without finish, droplet is seen; (b) For dyed and finished woven fabric, the water droplets do no spread or get absorbed by the surface; (c) For only dyed S/J knit fabric, droplet was absorbed; (d) Dyed and finished S/J fabric, the liquid could not penetrate through the fabric surface; (e) Finished S/J white fabric, the liquid could not penetrate through the fabric surface and for that reason, the liquid drop could not be seen |
2) Drop testThis is the visual test to evaluate the water repellency. If the material has lower surface tension than water, then that material is called water repellent. When a water droplet is placed on water repellent material then the drop will rest up on it and will not penetrate. Here are some physical appearances of untreated and treated fabrics, showed that treated fabrics are water repellent. These physical appearances were taken by using following configurationsCamera: Canon EOS 80DLens: 100mm Macro LensCamera settings: f/2.8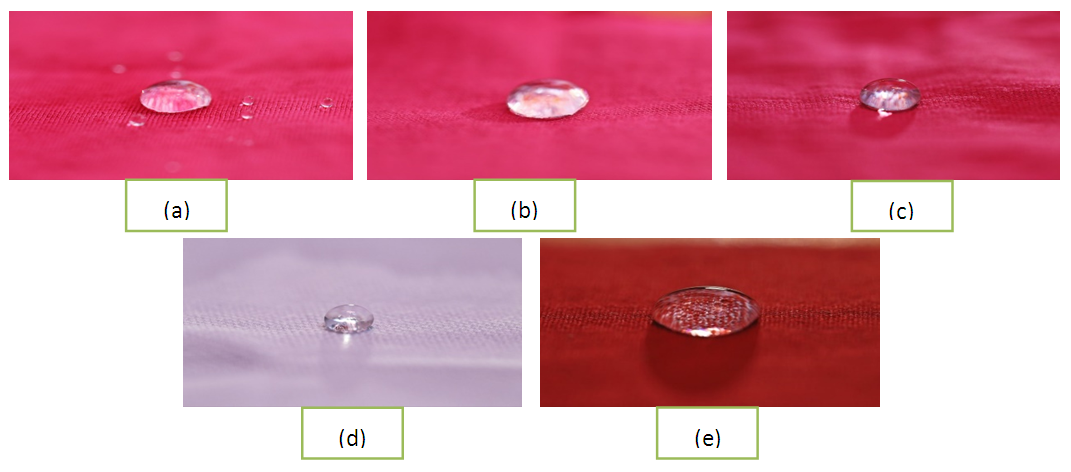 | Figure 4. Some physical appearances of water droplet on untreated and treated fabrics; (a) S/J fabric dyed and finished with FC+B; (b) S/J fabric dyed and finished with FC+D; (c) S/J fabric dyed and finished with FC; (d) S/J white finished fabric with FC+D; (e) woven dyed and finished fabric with FC+D water repellent chemicals |
3) Spray rating testFluorocarbon (FC), FC+dendrimers (FC+D) and FC+booster (FC+B) water repellent chemical’s solutions were prepared in 10 g/L, 30 g/L and 50 g/L concentrations and were applied on woven and S/J knit dyed fabrics with three different curing temperatures (140°C, 160°C and 180°C). There treated fabrics were evaluated by using AATCC 22-2001 method. The evaluated rating of the samples are given in chart in Figure 3. Schindler and Hauser described that by completing the pad-dry-cure process, the heat treatment changes perfluoro side chains to almost crystalline structures to achieve optimal water repellency [11]. Water repellencyof woven fabrics treated with fluorocarbon resin, with or without crosslinking agent, are shown in Table 4 and Figure 5 respectively. Compared with the untreated fabric, the finished fabrics had good water repellency with around 80-100 grades.Table 4. Spray rating of woven fabric treated with water repellent chemicals at 10 g/L, 30 g/L and 50 g/L conc. in different curing temperature
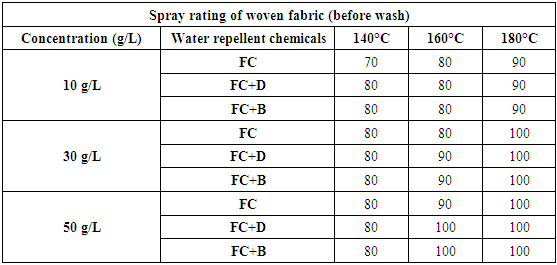 |
| |
|
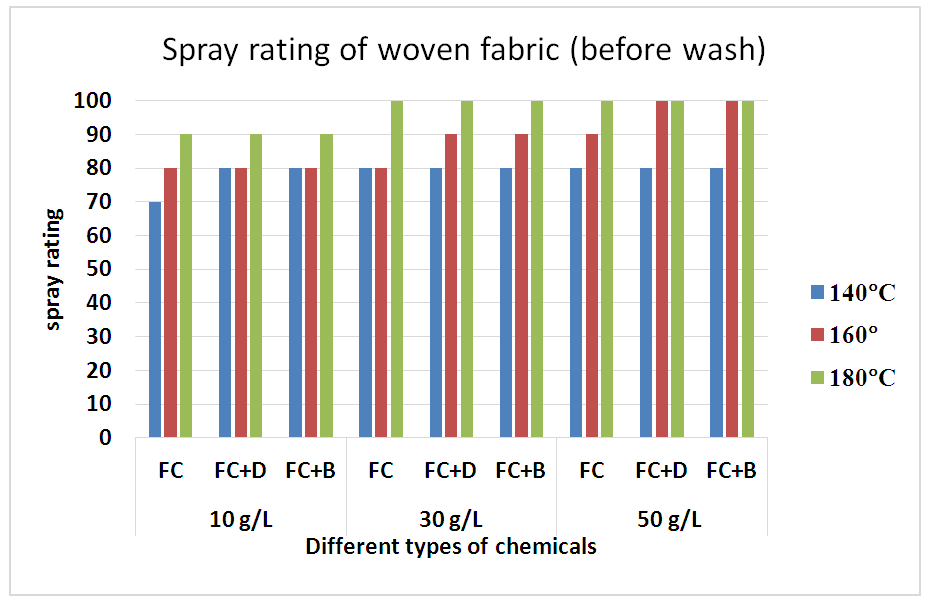 | Figure 5. Spray rating of woven fabric treated with water repellent chemicals at 10 g/L, 30 g/L and 50 g/L conc. in different curing temperature |
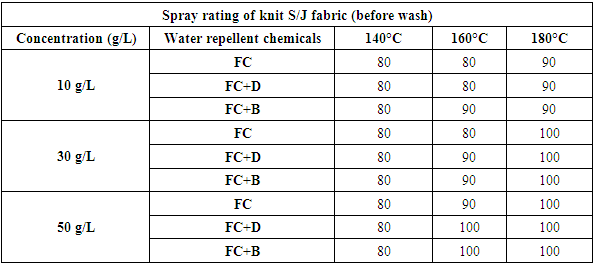
Water repellency of S/J fabrics treated with fluorocarbon resins with cross linking agent (FC+D and FC+B) or without crosslinking agent, are shown in Table 5 and Figure 6 respectively. Water repellency decreased considerably with washing and recovered to some extent with heat treatment. The decreas of water repellency for the fabric treated with the resin containing the cross linking agents (FC+B) and resin containing dendrimers (FC+D) are smaller than that of the fabric treated with resin (FC) alone, and recovered almost completely after heat treatment. Compared with the untreated fabric, the finished fabrics had good water repellency with around 80-100 grades. Both woven and S/J fabrics showed lower water repellency at 140°C curing temperature as it is assume that this curing temperature is not sufficient for proper cross linking of fluorocarbons. Table 5. Spray rating of knit single jersey fabric treated with water repellent chemicals at 10 g/L, 30 g/L and 50 g/L conc. in different curing temperature(before wash)
 |
| |
|
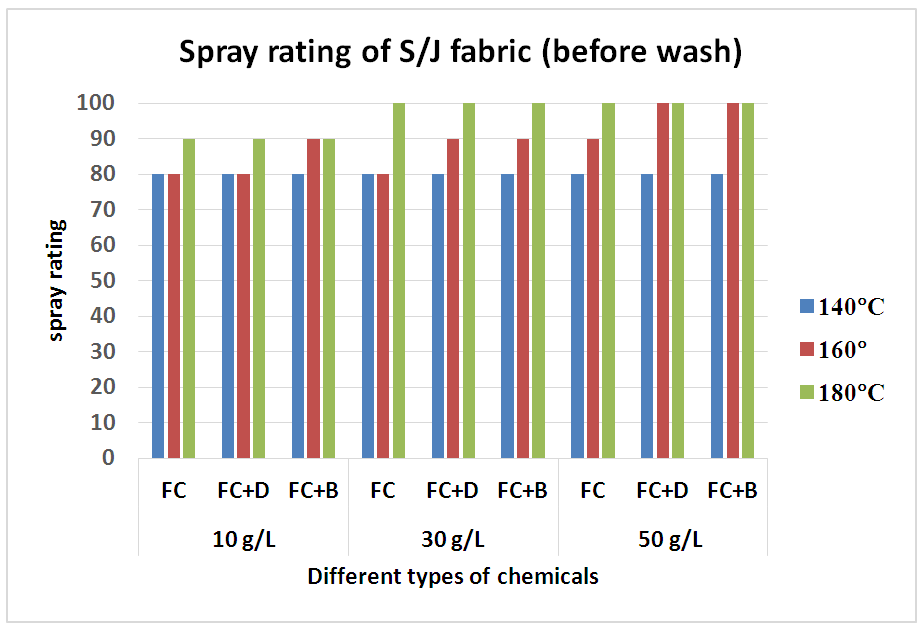 | Figure 6. Spray rating of knit S/J (single jersey) fabric treated with water repellent chemicals at 10 g/L, 30 g/L and 50 g/L conc. in different curing temperature(before wash) |
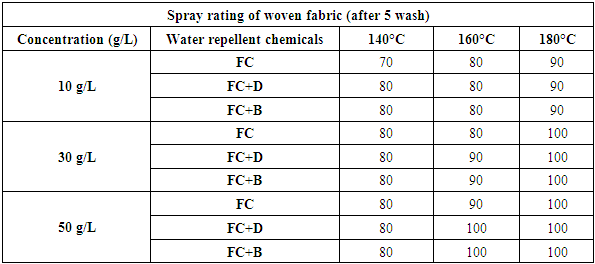
The durability of water repellency after repeated wash (after 5 wash) for woven fabric are shown in Tab. 6 and Fig: 7 and for S/J fabric are shown in Tab. 7 and Fig: 8 respectively. From these results, it is clear that adding 30 g/L and above than that concentrations (50 g/L) of resin treatment of cotton fabric are effective in suppressing the decrease in water repellency with washing. Usually, cellulose fibre swells in water and for that reason segments of cellulose molecules can easily move in the fibre during washing and drying.Table 6. Spray rating of woven fabric treated with water repellent chemicals at 10 g/L, 30 g/L and 50 g/L conc. in different curing temperature (after 5 wash)
 |
| |
|
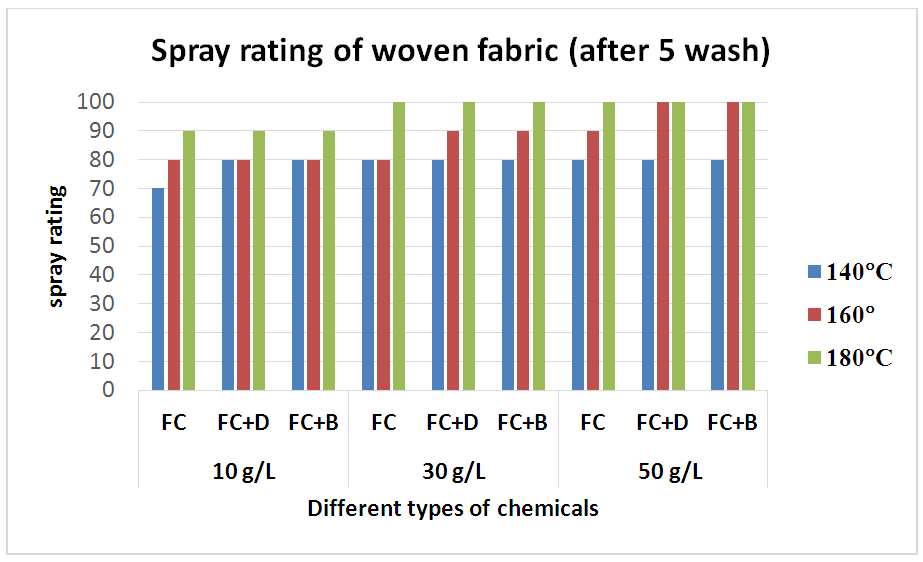 | Figure 7. Spray rating of woven fabric treated with water repellent chemicals at 10 g/L, 30 g/L and 50 g/L conc. in different curing temperature (after 5 wash) |
Table 7. Spray rating of S/J fabric treated with water repellent chemicals at 10 g/L, 30 g/L and 50 g/L conc. in different curing temperature (after 5 wash)
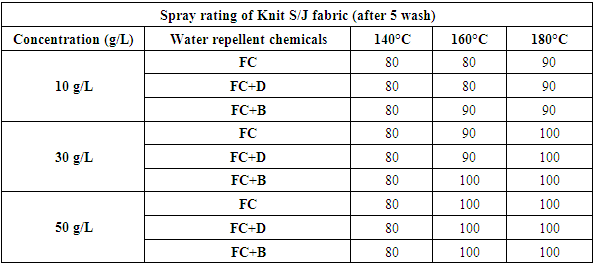 |
| |
|
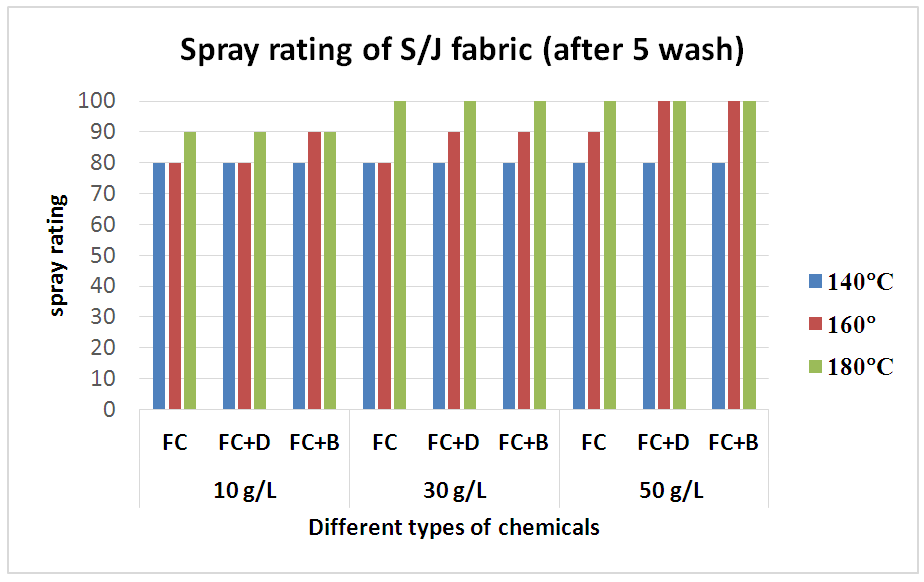 | Figure 8. Spray rating of S/J fabric treated with water repellent chemicals at 10 g/L, 30 g/L and 50 g/L conc. in different curing temperature (after 5 wash) |
Therefore, after treating with only fluorocarbon (FC) water repellent chemical, cellulose molecules chains of cellulose fibres are apt to move from the surface to the inner part of the fibre to avoid the hydrophilic conditions of washing, resulting a remarkable reduction in water repellency which is clearly evident from Table 8 and Figure 9 for woven fabric and Table 9 and Figure 10 for S/J fabric as well.Table 8. Spray rating of woven fabric treated with water repellent chemicals at 10 g/L, 30 g/L and 50 g/L conc. in different curing temperature (after 15 wash)
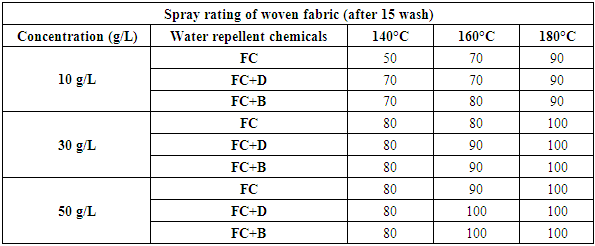 |
| |
|
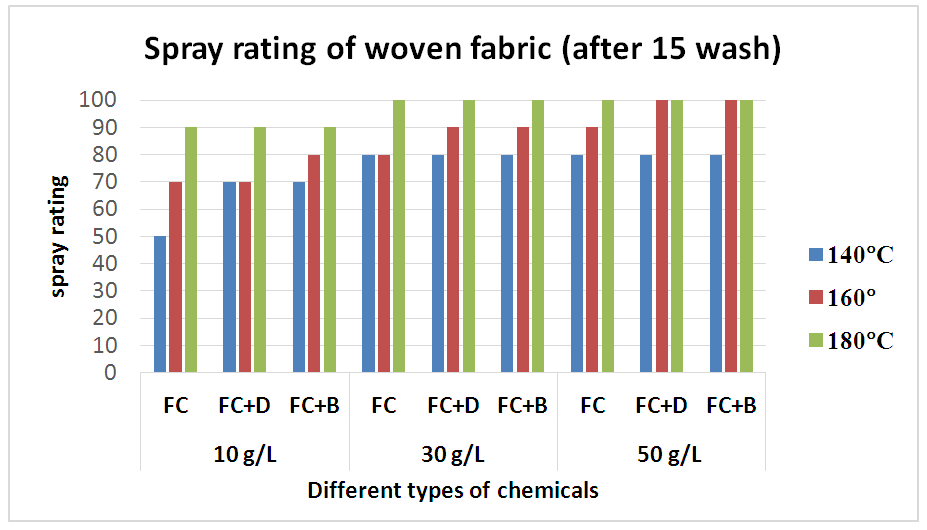 | Figure 9. Spray rating of woven fabric treated with water repellent chemicals at 10 g/L, 30 g/L and 50 g/L conc. in different curing temperature (after 15 wash) |
Table 9. Spray rating of knit S/J fabric treated with water repellent chemicals at 10 g/L, 30 g/L and 50 g/L conc. in different curing temperature (after 15 wash)
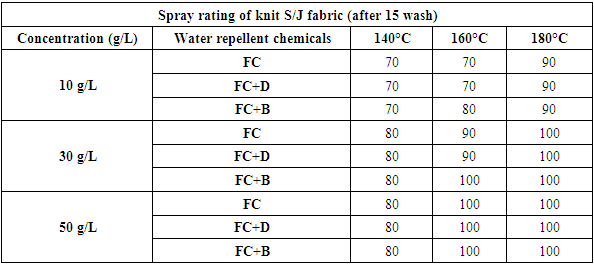 |
| |
|
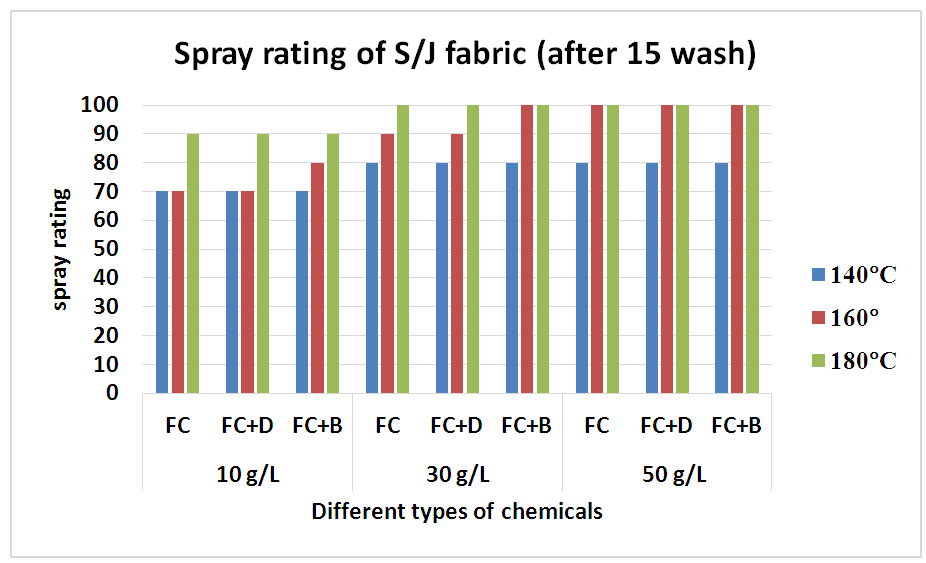 | Figure 10. Spray rating of knit S/J fabric treated with water repellent chemicals at 10 g/L, 30 g/L and 50 g/L conc. in different curing temperature (after 15 wash) |
On the other, fabric treated with FC+B and FC+D resins has better water repellency because cross linking between cellulose molecules or cellulose and fluorocarbon resins restrains the rotation of the fluoroalkyl groups into the fibre during washing.In 50 g/L concentration, water repellency rating of fluorocarbons for both woven and knit fabrics, we get better result comparatively with FC+B water repellent chemical for both fabrics with durability (15 wash) from 160°C and above curing temperature. In 30 g/L concentration, water repellency rating for both FC+D and FC+B water repellent chemicals above 160°C curing temperature show the best rating. Particularly for 10 g/L concentration and 140°C curing temperature, all fluorocarbons give the lowest results for all fabrics rather than others and in high concentration all the chemicals show better result for all fabrics with durability. In 10 g/L concentration and 140°C curing temperature, all fluorocarbons give the lowest results for all fabrics due to the lack of sufficient amount of resin and the proper heat treatment that are required to change perfluoro side chains to almost crystalline structures to achieve optimal water repellency [11].4) Hydrostatic head testThe hydrostatic head test was done according to AATCC 127 method of woven and S/J fabrics after water repellent finish at various concentration which are stated below in Table 10 and Figure 11.Table 10. The hydrostatic head test of woven fabrics treated with water repellent chemicals at 10 g/L, 30g/L and 50g/L conc. at different curing temperature
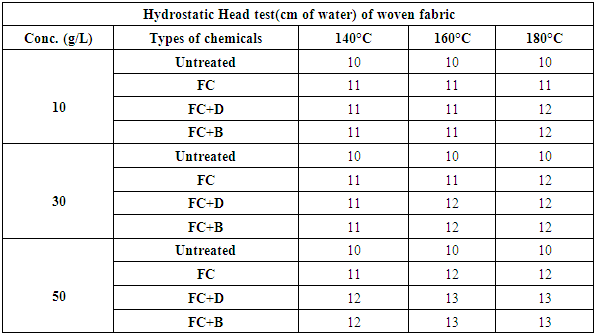 |
| |
|
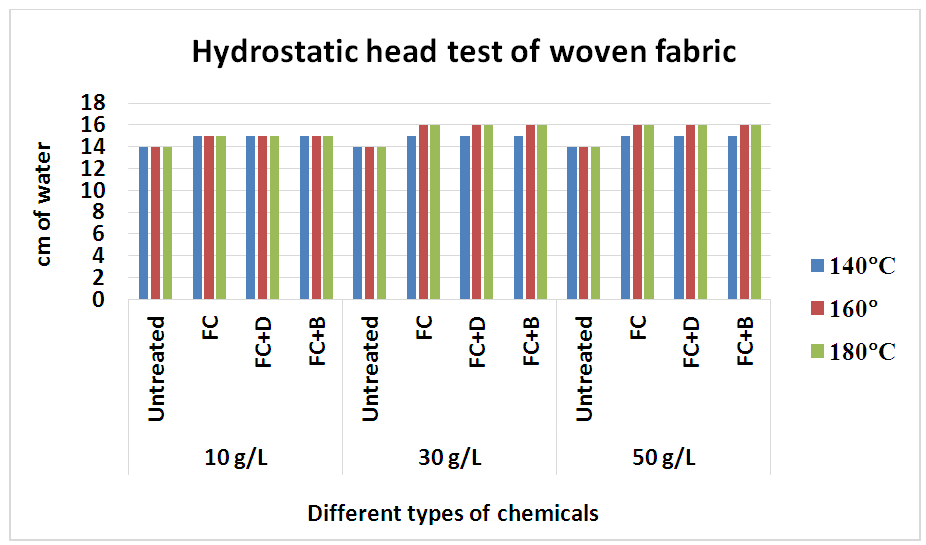 | Figure 11. The hydrostatic head test of woven fabrics treated with water repellent chemicals at 10 g/L, 30g/L and 50g/L conc. at different curing temperature |
Table 11. The hydrostatic head test of S/J fabrics treated with water repellent chemicals at 10 g/L, 30g/L and 50g/L conc. at different curing temperature
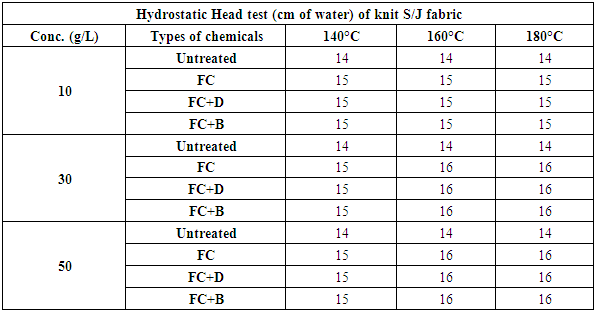 |
| |
|
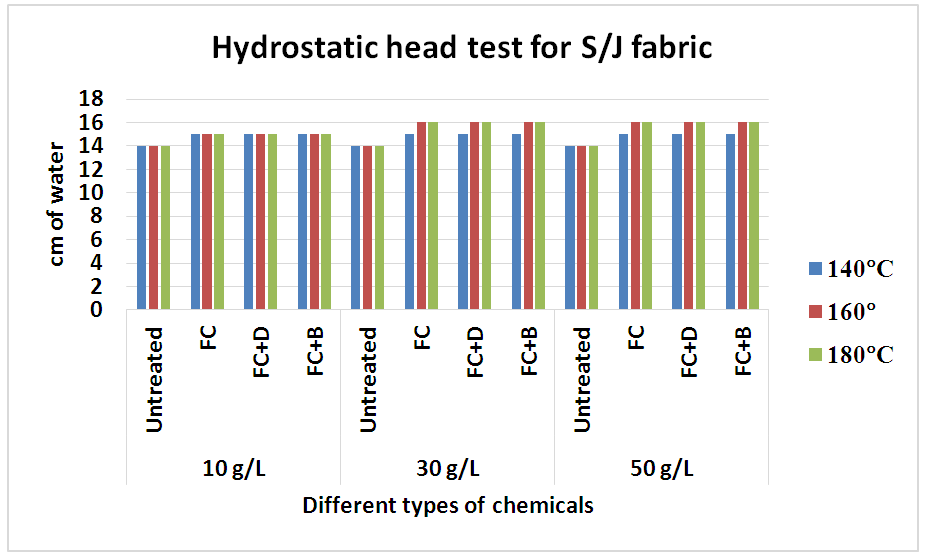 | Figure 12. The hydrostatic head test of knit S/J fabric treated with water repellent chemicals at 10 g/L, 30 g/L and 50 g/L conc. |
This test is basically done for high density fabric like canvas or umbrella fabric. From the above chart it has seen that the more pressure required to force water through the fabric and with the concentration increases, the pressure requires more and gradually from 30 g/L to 50g/L. An increase in repellent concentration caused an increase in wetting times of the fabric after finishing. Because the water repellent chemicals form a coating on the fabric and the more concentration of chemicals leads the higher density of the coating resulting increased water repellency.
3.2. Analysis of GSM
The GSM tests are done according to ASTM D 3776-79 method for all fabrics with different concentration at fixed 160°C curing temperature. The variation of GSM after finishing has given in below Table 12. and Figure 13.After chemical implementation of woven and knit fabrics with various concentrations, GSM has increased because chemical has covered up all the pores of the fabric and a chemical coating is created on the fabric. Therefore, the water is not allowed to penetrate into the fabric.Table 12. GSM of fabrics treated with water repellent chemicals at 10 g/L, 30g/L and 50g/L conc.
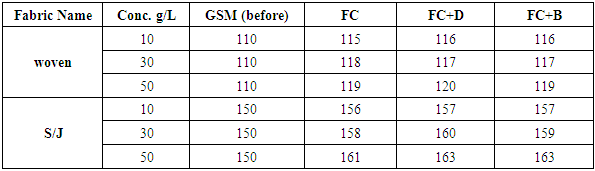 |
| |
|
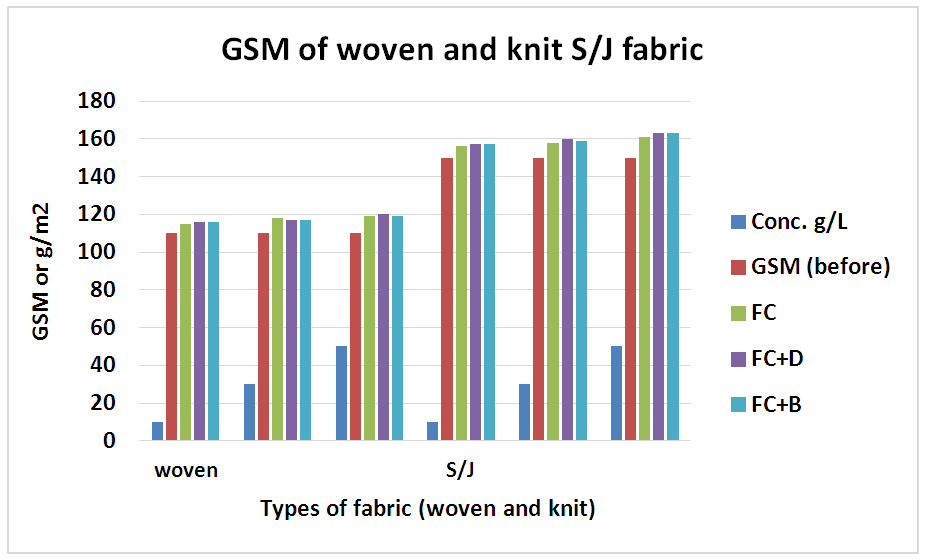 | Figure 13. GSM of different fabrics treated with water repellent chemicals at 10 g/L, 30 g/L and 50g/L conc. |
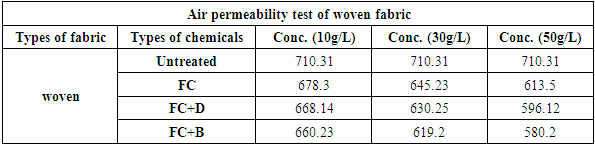
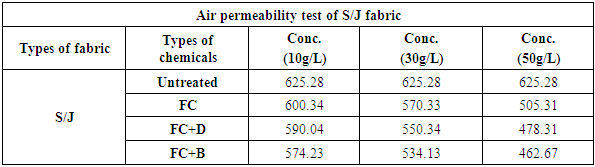
3.3. Air Permeability Test
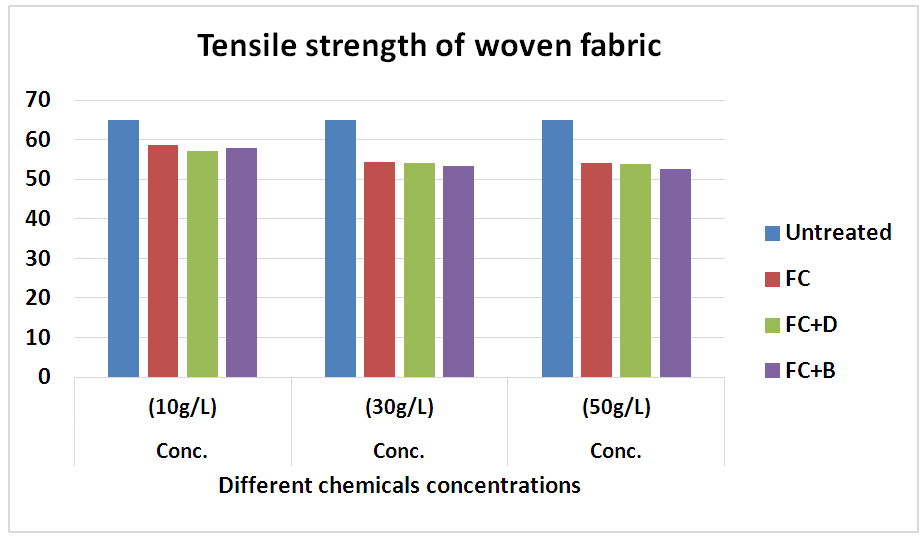
It was done by air permeability tester by using ISO 9237 method for all fabrics with different concentration at fixed 160°C curing temperature. The air permeability of the fabrics decreased via water repellent finish. This may be because of the formation of cross linking networks after finish. The thin film formation on the surface of fabric after finishing, tighter constructions and small pore dimensions are some of the factors that affect the lower air permeability. In 10 g/L conc., water repellency rating of FC for both woven and knit fabrics, we get better result comparatively than FC+D and FC+B water repellent chemicals.In 30 g/L and 50 g/L conc., air permeability rating for both FC+D and FC+B are lower than FC water repellent chemical. As in higher (30 g/L and 50 g/L) conc. all fluorocarbons give the highest results for all fabrics due to the lack of sufficient amount of resin and the proper heat treatment that are required to form film formation. Tensile Strength Test1) Tensile strength plays a vital role after water repllent finish. It was done according to ASTM (D 5045-87) method to evaluate the treated woven fabric’s tensile strength. In here, in the chart the tensile strength of the cotton woven fabric shows slight detoriation occurs and it is obviously taken into account as resin crosslinks in amorphous region of cellulose leading lower flexibility and harsh handfeel. Though it is marginal for the fabric to go for the next proceedings. Table 13. Air permeability test rating of woven fabric treated with water repellent chemicals at 10 g/L, 30 g/L and 50 g/L conc.
 |
| |
|
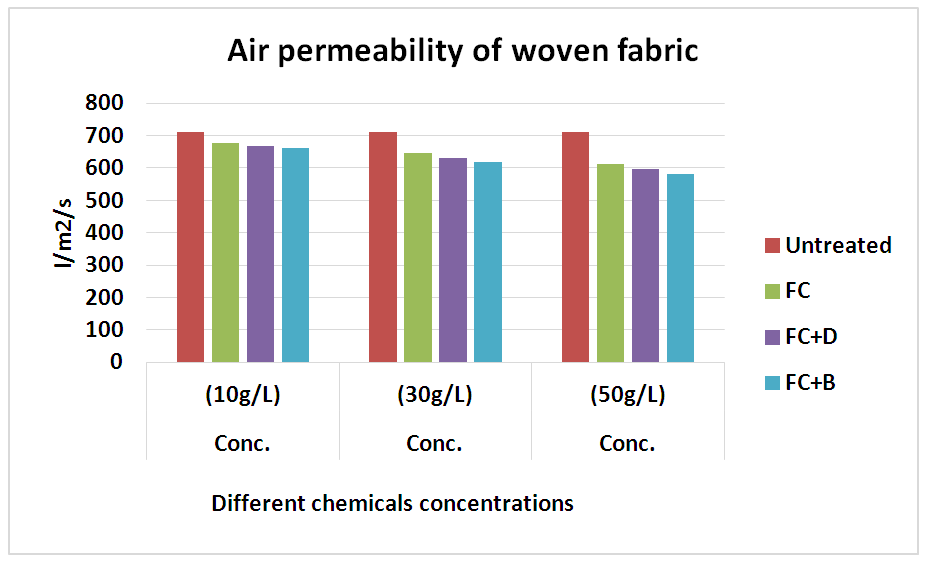 | Figure 14. Air permeability rating of woven fabric treated with water repellent chemicals at 10 g/L, 30 g/L and 50 g/L conc. |
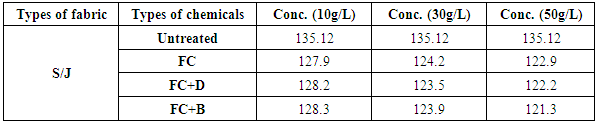
Table 14. Air permeability rating of S/J fabric treated with water repellent chemicals at 10 g/L, 30 g/L and 50 g/L conc.
 |
| |
|
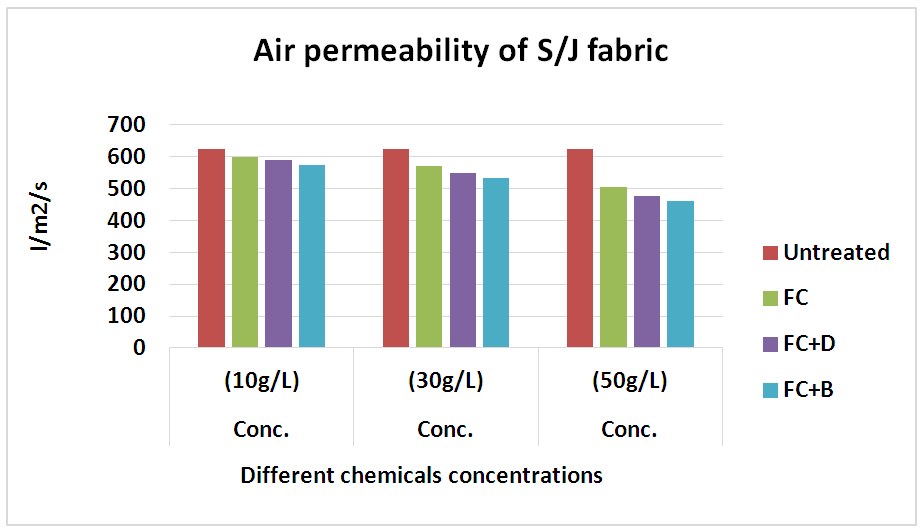 | Figure 15. Air permeability rating of S/J fabric treated with water repellent chemicals at 10 g/L, 30 g/L and 50 g/L conc. |
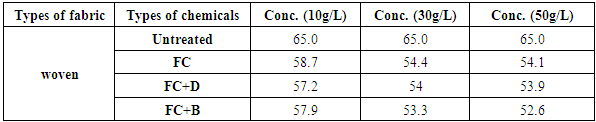
3.4. Tensile Strength of Woven Fabric
Table 15. Tensile strength of woven fabric treated with water repellent chemicals at 10 g/L, 30 g/L and 50 g/L conc.
 |
| |
|
 | Figure 16. Tensile strength of woven fabric treated with water repellent chemicals at 10 g/L, 30 g/L and 50 g/L conc. |
3.5. Bursting Strength for S/J fabric
It was done by Trust brust tester according to ASTM (D 3786-87) method. From the above chart in Figure 17, it is clear that after water repellent finish the fabric strength is decreased and it’s reasonable. The fabric strength is decreased by increasing of concentration. The bursting strength of knit fabric reduced because of effect of the cellulosic fiber during cross linking process. When the water repellent chemicals form cross link with the cotton free O-H group in the amorphous region, it makes stiff of the fabric and moreover, cross linking reaction is done mainly in acidic condition which are also responsible for the loss of the fabric bursting strength.Table 16. Bursting strength test of S/J fabric treated with water repellent chemicals at 10 g/L, 30 g/L and 50 g/L conc.
 |
| |
|
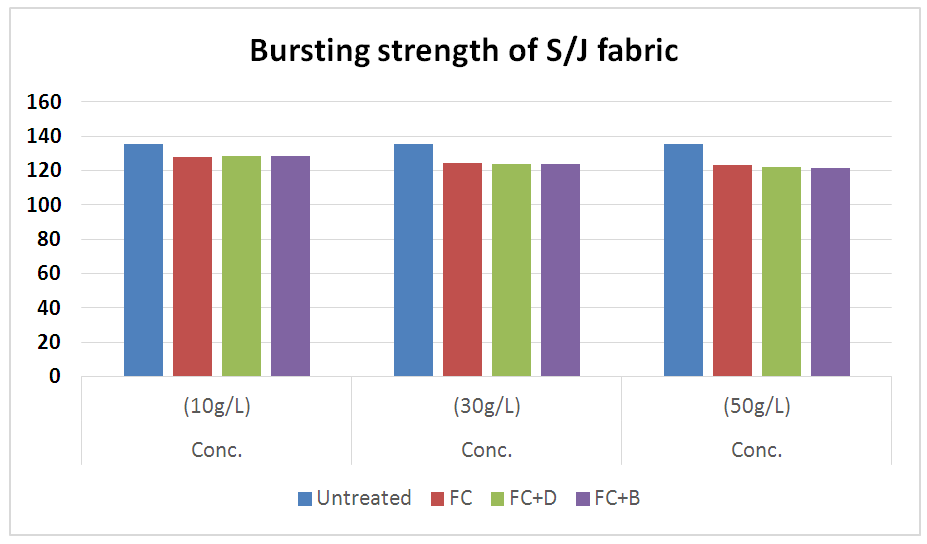 | Figure 17. Bursting strength test of S/J fabric treated with water repellent chemicals at 10 g/L, 30 g/L and 50 g/L conc. |
3.6. Abrasion Resistance
The abrasion resistances of the treated fabrics were determined with the weight decreasing rate. If the weight decreasing rate is less, it indicates the good abrasion resistance of the fabric and vice versa. The result was shown in Table 17 and Figure 18 for woven fabric and in Table 18 and Figure 19 for S/J fabrics with different concentration at fixed 160°C curing temperature.It could be seen that the abrasion resistance of the finished cotton fabric increased. This may be for the formation of the film on the cotton fabric after crosslinking of water repellent resins. For woven fabric, FC+D and FC+B both water repellent chemicals show good abrasion resistance than FC in all concentrations. On the other hand, 30 g/L and 50 g/L concentrations of all water repellent chemicals show better abrasion resistance than 10 g/L concentration. Because the density of the fabric was increased with the formed film, and the floating length of the yarn was shorter. It made the stiff nodal point formed with the sustaining point, the fibres squeezed each other during the abrasion process, which affected the relative movement. The stress centralized made the damage of the fabric. Table 17. Abrasion resistance of woven fabric treated with water repellent chemicals at 10 g/L, 30 g/L and 50 g/L conc.
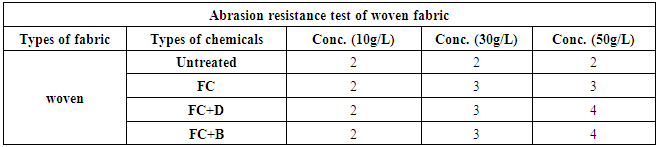 |
| |
|
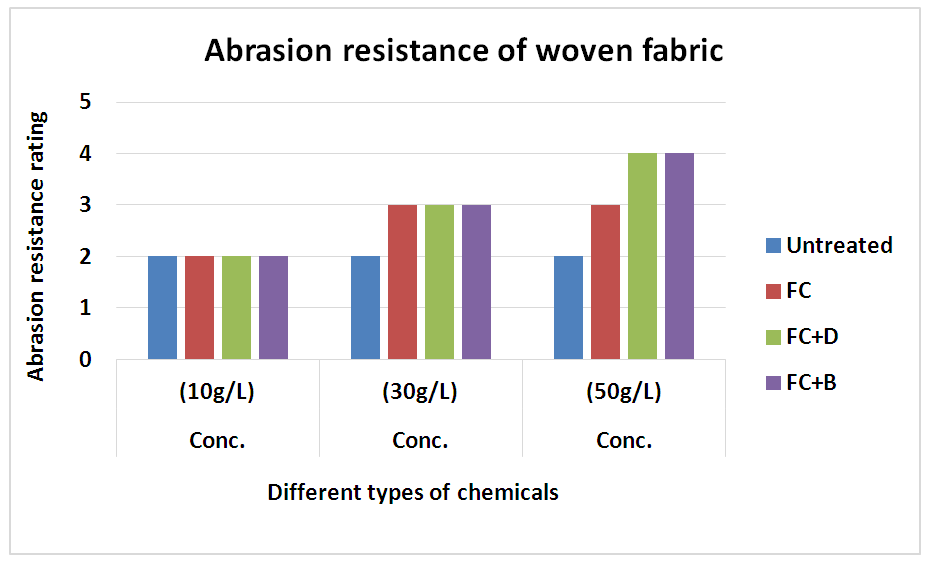 | Figure 18. Abrasion resistance of woven fabric treated with water repellent chemicals at 10 g/L, 30 g/L and 50 g/L conc. |
Table 18. Abrasion resistance of S/J fabric treated with water repellent chemicals at 10 g/L, 30 g/L and 50 g/L conc.
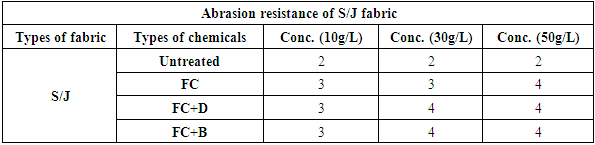 |
| |
|
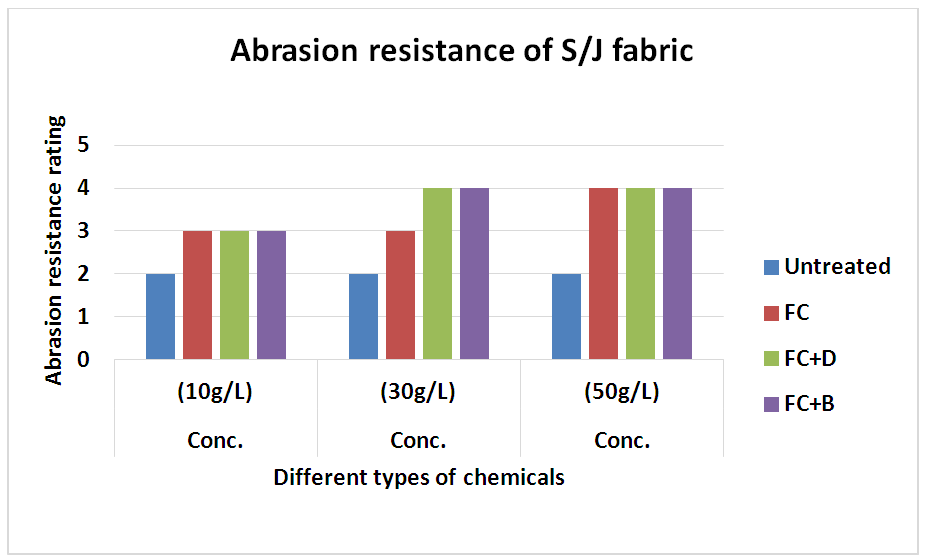 | Figure 19. Abrasion resistance of S/J fabric treated with water repellent chemicals at 10 g/L, 30 g/L and 50 g/L conc. |
3.7. Color Fastness to Wash
To evaluate the effect of wash fastness of water repellent treated fabrics are rated under grey scale for two types of measurement, one is for color change and another is for color staining.Color fastness to wash was measured with ISO 105 C03 method. The rated chart is given below in Table 19. At first the sample is washed with standard recipe and then color change is measured to the washed sample with the unwashed sample and compared. Color staining is also measured with grey scale with the help of multifibre.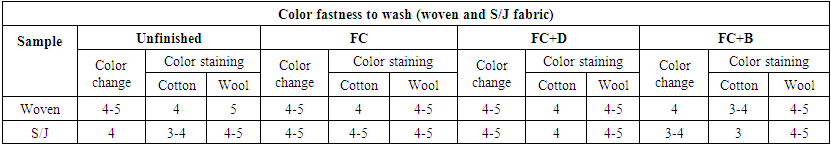 | Table 19. Color Fastness to wash of different fabrics treated with water repellent chemicals at 50g/L conc. |
The wash fastness of all water repellent chemicals were measured at 50 g/L concentration (conc.) as it showed the best water repellency and physical properties compared with other concentrations. The wash fastness of FC and FC+D water repellent chemicals shows better result than FC+B chemicals for both woven and S/J fabrics at 50 g/L conc. The improvement of wash fastness of water repellent finish is because of the dye molecules trapped inside the crosslinking film.
3.8. Color Fastness to Water
To evaluate the effect of water fastness of water repellent treated fabrics are rated under grey scale for two types of measurement, one is for color change and another is for color staining. Color fastness to wash was measured with ISO 105 E01 method. The rated chart is given below in Table 20. The water fastnesses of all water repellent chemicals were measured at 50 g/L conc. The water fastness of FC and FC+D water repellent chemicals shows better result than FC+B chemicals for both woven and S/J fabrics at 50 g/L conc. 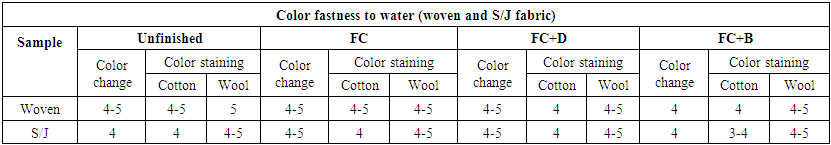 | Table 20. Color Fastness to water of different fabrics treated with water repellent chemicals at 50g/L conc. |
3.9. Color Fastness to Perspiration
The resistance of color against acid and alkali of dyed fabrics were done by ISO 105-E04 method. The rated chart is given below in Table 21. The fastness to perspiration of all water repellent chemicals were measured at 50 g/L conc. The fastness to perspiration of FC water repellent chemical shows better result than FC+D and FC+B chemicals for both woven and S/J fabrics.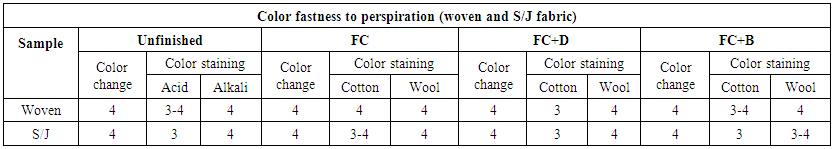 | Table 21. Color Fastness to perspiration of different fabrics treated with water repellent chemicals at 50g/L conc. |
3.10. Rubbing Fastness
The resistances of color against rubbing of dyed fabrics (dry and wet) were evaluated with ISO-105-X 12 method. The rubbing fastness of water repellent treated fabric with different concentration is rated under grey scale for the measurement of color staining against dry and wet white fabric. The rated chart of rubbing fastness for wet and dry rub is given below in Table 22. It shows that both dry and wet rub are increased after water repellent finish on conc. 50 g/L as water repellent chemical make a thin coating on fabric surface.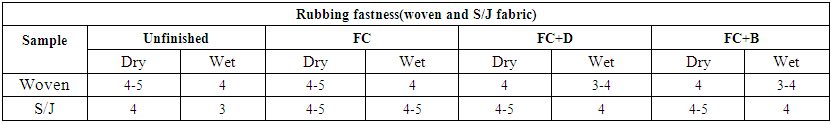 | Table 22. Rubbing fastness of different fabrics treated with water repellent chemicals at 50g/L conc. |
From the data sheet (staining), it is found that for all three FC, FC+D and FC+B chemicals shows good rubbing (dry and wet) fastness than untreated ones for S/J fabrics. Whereas wet and dry rub staining for woven fabric shows slightly decreased for both FC+D and FC+B than untreated one, but it remain same for FC water repellent finish
4. Conclusions
In this study, the effect of fluorocarbon based water repellent finishes with varying concentrations and varying curing temperature were observed on the water repellency of 100% cotton (woven and knit) fabrics. To do so, 54 repellent treated fabrics samples were tested and obtained results were evaluated. Different physical and chemical test results showed that the repellent types and their concentration ranges significantly influenced water repellency of both woven and knit fabrics. When fluorocarbon with dendrimers (FC+B) was used, then the best water repellency with durability are obtained and this finish’s durability was evaluated by repeated laundering. Changing concentration and curing temperature from lower to higher level, gives gradually increased water repellency, regardless of repellent chemical type. Water repellency was evaluated by drop test, contact angle, spray rating and hydrostatic head test. However, unlike the findings of previous research works which described that if the water repellent was used with higher concentration, strength is decreased. The repellent chemicals and their changing concentrations did not cause significant change to bursting strength of knit fabrics. But higher curing temperature had cause strength loss. Higher concentration of chemicals gives increased abrasion resistance but decreased airpermeability. There was no remarkable deviation is observed in GSM on the basis of water repellent chemicals and their varying concentrations. A higher in repellent concentration caused a satisfactory wash, water, perspiration and rubbing fastness. The coloring finishes had no effect on water repellency is another important conclusion of this work.
ACKNOWLEDGEMENTS
I would also like to express my deep gratitude to MICRO FIBRE for providing us the lab facilities, essentially used during the work. I would also like to thank Essential Clothing Ltd for providing me knit fabrics. Again special gratitude also goes to the Wet Processing Lab & the TTQC Lab of Bangladesh University of Textiles (Butex) for most of the physical tests.
References
| [1] | Bongiovanni R, Zeno E, Pollicino A, Serafini PM, Tonelli C, “UV-light induced grafting of fluorinated monomer onto cellulose sheets”, Cellulose, 2011, 18, 117-126. |
| [2] | Ferrero E, Periolatto M, Udrescu C, “Water and oil-repellent coatings of perfluoro-polyacrylate resins on cotton fibres: UV curing in comparison with thermal polymerization”, Fibre. Polym, 2012, 13, 191-198. |
| [3] | Xu CH, Jia ST, Zhang J, Ma JZ, “large-area fabrication of superhydrophobic surfaces for practical applications: An overview”, Sci. Technol. Adv. Mat., 2010, 11, 1-15. |
| [4] | Castelvetro V, Francini G, Ciardelli G, Ceccato M, “Evaluating fluorinated acrylic lattices as textile water and oil repellent finishes”, Text. Res. J, 2001, 71, 399-406. |
| [5] | Shao H, Sun J Y, Meng W D, Qing F L, “Water and oil repellent and durable press finishes for cotton based on a perfluoroalkyl-containing multi-epoxy compound and citric acid”, Text. Res. J, 2004, 74, 851-855. |
| [6] | Lee H J, Michielsen S, “Preparation of a superhydrophobic rough surface”, J. Polym. Sci. Part B, Polym. Phys, 2007, 45, 253-261. |
| [7] | Li Z R, Fu K J, Wang L J, Liu F, “Synthesis of a novel perfluorinated acrylate copolymer containing hydroxyethyl sulfone as crosslinking group and its application on cotton fabrics”, J. Mater. Process. Technol, 2008, 205, 243-248. |
| [8] | Roe B, Zhang X, “Durable hydrophobic textile fabric finishing using silica nanoparticles and mixed silanes”, Text. Res. J, 2009, 79, 1115-1122. |
| [9] | Kasturiya N, Bhargava GS, “Liquid repellency and durability assessment: A quick technique”, J. Ind. Text., 2003, 32, 187-222. |
| [10] | Mohsin M, Sarwar N, Ahmed S, Rasheed A, Ahmad F, Afzal A, Zafar S, “Maleic acid crosslinking of C-6 fluorocarbon as oil and water repellent finish on cellulosic fabric”, J. Chem. Prod, 2016, 112, 3525-3530. |
| [11] | Schindler WD and Hauser PJ, “Chemical finishing of textiles”, Cambridge: Woodhead Publishing Ltd, 2004, p. 80-82. |
| [12] | Sahin B, “Fluorochemicals in textile finishing”, Int. Text. Bull.-Dyeing/Printing/Finishing, 1996, 42(3), 26-30. |
| [13] | Corpat J M, Dessaint A, “Fluorine-based Textile Finishes, Melliand Textilber. Eng, 1997, 78(9), E135-E137. |
| [14] | Grottenmuller R, “Fluorocarbons-An Innovative Aid to the Finishing of Textiles, Melliand Int. 1998, 4, 278-281. |
| [15] | Hangjin Q, Kunyan S, Zhaoli M, Dong W, Xiquan S, Jianjun Lu, “Polymeric Fluorocarbon Coated Polyester Substrates for Waterproof Breathable Fabrics”, Textile Res. J. 2002, 72(2), 93-97. |























 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML


















