-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Textile Science
p-ISSN: 2325-0119 e-ISSN: 2325-0100
2018; 7(2): 43-47
doi:10.5923/j.textile.20180702.02

Microbial Keratinase Production and Application to Improve the Properties of Wool Fabrics
G. Berrak Gunes1, Orkan Akkoyun1, Tugce Demir2, Ebru Bozaci3, Asli Demir3, E. Esin Hames2
1Department of Biotechnology, Graduate School of Natural and Applied Science, Ege University, Izmir, Turkey
2Department of Bioengineering, Faculty of Engineering, Ege University, Izmir, Turkey
3Department of Textile Engineering, Faculty of Engineering, Ege University, Izmir, Turkey
Correspondence to: E. Esin Hames, Department of Bioengineering, Faculty of Engineering, Ege University, Izmir, Turkey.
| Email: |  |
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Keratinases have been used because of their ability to separate keratin that cannot be broken down by common proteases in the processing of wool fabrics in the textile industry. In this study, to enhance the keratinase production from Streptomyces sp. 2M21, three different detergents (SDS, Triton X-100, Tween 80) in two different concentrations (0.5%, 0.1%) were added to the fermentation medium containing raw sheep wool as a solid substrate and results were evaluated compared to the control groups. The highest enzyme activity was obtained from 0.5% Tween 80 containing fermentation medium that increased enzyme activity from 111 (U/mL) to 399 (U/mL). Subsequently, the produced keratinase and commercial enzyme were compared for improving the hydrophilicity, dyeing and shrinkage properties of wool fabrics. Results suggest that, the keratinase produced from raw wool is feasible for processing of wool fabrics in textile industry.
Keywords: Keratinase, Streptomyces sp., Solid substrate, Wool fabric, Textile finishing process
Cite this paper: G. Berrak Gunes, Orkan Akkoyun, Tugce Demir, Ebru Bozaci, Asli Demir, E. Esin Hames, Microbial Keratinase Production and Application to Improve the Properties of Wool Fabrics, International Journal of Textile Science, Vol. 7 No. 2, 2018, pp. 43-47. doi: 10.5923/j.textile.20180702.02.
Article Outline
1. Introduction
- Keratin is a fibrous, insoluble and high stable protein [1], especially found in skin, hair, wool, nail, feather and beak of birds; shells and scale of reptiles [2]. Depending on their secondary structural conformation, keratins are divided into two main groups that are α (hair and wool) and β (feather) keratin. Keratin is also categorized as hard and soft keratin comprising the amount of sulphur in their amino acids content. Soft keratin (skin and callus) is less resistant to chemical agents and degradation process comparing to hard keratin (feather, wool, nails) because of their low cysteine content [3]. Wool structure composed of mainly keratinous protein (82%) and non-keratinous protein (17%). Small amount (1%) waxy lipid and polysaccharide are also found in wool [4, 5].Keratinases [EC 3.4.21/24/99.11] degrade the keratins, are an important group of proteolytic enzymes which are mainly belong to serine or metallo proteases [6]. Common proteolytic enzymes such as pepsin, trypsin and papain cannot cleave keratins due to their rigid structure. Keratinases produced by some microbial species are responsible for the degradation of keratinous substrates in the nature [7]. In fungi, keratinase producers are generally relating to dermatophytes comprising Microsporum, Trichophyton, Chrysosporium, Geomyces [8]. In bacteria, mostly Gram positive group (Bacillus, Lysobacter, Nesternokia, Kocurica, and Microbacterium) come to the forefront for keratinase production [6]. There are also known promising keratinase producers in actinomycetes especially from Streptomyces genus [9, 10], Streptomyces fradiae [11], Streptomyces pactum [12], Streptomyces graminofaciens [13], Streptomyces gulbarguensis [14] and Thermoactinomyces sp. [15] can be given as keratin hydrolysing actinomycetes. The properties of keratinase depend to the producer organism. In general, this enzyme is produced extracellularly, and catalysis occurs in neutral and alkali pH values and wide temperature range [6]. Proteases are commonly used for the enzymatic treatment of wool fabrics in the textile industry. These enzymes enhance hydrophilicity, dyeing and shrinkage properties, impart anti-shrinkage/anti-felting features to the wool fabrics [16]. Importance of keratinase is also growing for various industries such as feed, fertilizer, detergent, leather, pharmaceutical and biomedical industry [17]. The wool fiber has hydrophobic properties by the reason of exo and epicuticle layers. Exocuticle contains 35% of disulphide cross linkage whereas epicuticle consists of fatty acid (25% by mass) and protein (75% by mass). Felting and shrinkage are the major problems for wool fabrics. Chemical methods are commonly used to eliminate the scales on the wool surface [4, 5]. However, these methods cause decrease in the strength of the fibers and non-uniform properties on the wool surface [18]. Enzymatic treatment is an alternative for improving the quality of wool fabrics [4, 5]. The mostly used enzymes, keratinase and protease, preferentially attack the disulphide cross linkages between fibers imparting hydrophilicity, dyeing and shrink resistance to wool fabrics. The aim of the study was enhancing keratinase production from Streptomyces sp. 2M21 by using sheep wool as a substrate with the aid of different surface-active compounds. Subsequently, the application potential of the keratinase in wool processing was determined by comparison with commercial protease in terms of hydrophilicity, dyeing and shrinkage properties.
2. Materials and Methods
2.1. Microorganism
- Isolate Streptomyces sp. 2M21 which is known as keratinase producer, from Actinomycetes Culture Collection (ACTINOCC) founded by Bioengineering Department of Ege University, registered to World Federation of Culture Collection (No. 952) was used in this study.
2.2. Preparation of Inoculum
- Streptomyces sp. 2M21 was inoculated onto soy flour mannitol agar (SFM) consisting of (g/L) soy flour 20, mannitol 20, agar 20, (pH 8.0) and incubated at 28C (Nüve ES500, Turkey) for 5-8 days. Mature spores (8x108) were transferred to 5 ml of Glycerol yeast extract broth (GY) consisting of (g/L) peptone 25, yeast extract 2, glycerol 5, K2HPO4 0.1 and incubated in water bath at 50C for 5 minutes to accelerate the germination of the spores prior to fermentation experiments [10].
2.3. Enhancing Keratinase Production Using Surface-active Compounds
- Raw sheep wool supplied from local butcher was used as a solid substrate for the production of keratinase from Streptomyces sp. 2M21. To facilitate the usage of wool by bacteria and enhancing the keratinase yield, three surface-active compounds, namely sodium dodecyl sulphate (SDS), triton X-100 and tween 80 were added to the medium in two different concentrations (0.1% and 0.5%). All fermentation experiments were done in 250 ml Erlenmeyer flasks with 50 ml of mineral salt medium (g/L: CaCO3, 5; MgSO4.7H2O, 0.2; K2HPO4, 3; NaCl, 5; pH 8.0) supplemented with wool (5 g/L) as a carbon and nitrogen source. Germinated spores were inoculated aseptically to the fermentation medium and incubated at 28°C, 150 rpm for 11 days. All fermentation experiments were carried out in duplicate. Enzyme production was monitored according to the keratinase activity. At the end of the fermentation, the remaining solid material was removed after centrifugation at 5.000 rpm for 2 min (Hettich Universal, 32) for further enzymatic treatment experiments.
2.4. Enzyme Activity Assay
- Keratinase activity was measured according to DelMar et al. [19]. Briefly, synthetic peptide, Suc-Ala-Ala-Pro-Phe-NA (BaChem) was dissolved in mixture of 0.05 M Tris-HCl buffer (pH 8) and dimethyl sulfoxide (DMSO) (Merck Chemicals) [1:99 (v/v)] used as a substrate solution. The reaction mixture [140 µl of 0.05 M Tris-HCl buffer (pH 8), 50 µl of 2mM substrate solution and 10 µl of cell free fermentation broth] was incubated at 37°C for 10 minutes. Amount of released pNA (p-nitroanilide) was measured by using spectrophotometer (VersaMax, USA) at 405 nm against the blank which was consisted of 150 µl of Tris-HCl buffer and 50 µl of substrate solution. Each measurement was performed in triplicate. One unit of keratinase activity was defined as the amount of enzyme required to liberate 1 μmol pNA under standard conditions.
2.5. Textile Application of the Keratinase
- Wool fabrics (with a mass of 245 g/m2 100 % pure, gabardine) supplied by Yünsa Company-Turkey was used in the experiments. The dyes used were kindly supplied by DyStar Company and the commercial protease enzyme was supplied by Novozyme.
2.5.1. Enzymatic Treatment
- Commercial protease and keratinase treatments were (400 U/mL) applied by the exhaustion method for 1 hour at a liquor ratio of 30:1 using an ATAC LAB DYE HT 10 (Ataç, Turkey) machine at 55°C for protease and 37°C for keratinase and pH 8 with 0.5 g/L non-ionic surfactant. Following the enzymatic treatment, the fabrics were rinsed with water at pH 4, 40°C and kept at for 10 minutes to denature the enzyme. Finally, the fabric was rinsed several times with deionized water to remove any remaining enzyme from the treatment.
2.5.2. Dyeing
- The dye used in this study was Realan Rot G and Realan Blau RC (wool reactive dye, pH 4.5 - 5). The liquor to fabric ratio was 10:1 (v/w). Subsequently dyeing, the fabrics were rinsed with water and then dried at room temperature.The K/S values of the dyed fabrics were measured by HunterLab ColorQuest II spectrophotometer instrument between 390 – 700 nm wavelength. The reflectance values (R) were measured and relative colour strength (K/S) values were then established according to the following Kubelka–Munk equation: K/S=[(1 − R)2/2R], where K and S are the absorption and scattering coefficients, respectively. The dyeing graph of Realan Rot RC and Realan Blau RC dyes were shown in Figure 1.
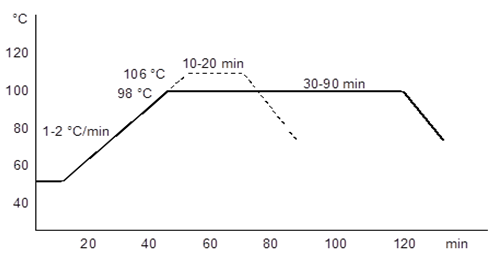 | Figure 1. The dyeing graph of Realan Rot G and Blau C |
2.5.3. Hydrophilicity
- The hydrophilicity (wettability) of fabrics was measured according to AATCC 79-1995 standard. A drop of water was allowed to fall from a fixed height onto the taut surface of a test specimen. The time required for the water drop to disappear was measured and recorded as wetting time. All experiments were carried out five times.
2.5.4. Determination of Shrinkage
- Wash shrinkage tests were carried out in a Wascator using the ISO 6330 5A programme, as described in IWS Test Method 31. All experiments were carried out five times.
2.5.5. Surface Observation
- For surface observation, changes on the fabric surface were evaluated using scanning electron microscopy (SEM). SEM observations were made with a Phillips XL-30S FEG scanning electron microscope.
3. Results
3.1. Enhancing the Keratinase Production
- In the first part of this study the keratinase production from Streptomyces sp. 2M21 was enhanced. Three different surface-active compounds were added to the fermentation medium in two different concentrations and the growth of microorganism and enzyme activity were monitored. Tween 80 in two different concentrations (0.5% and 0.1%) enhanced the keratinase production of Streptomyces sp. 2M21. However, different concentrations of the SDS and triton X-100 were not supplied microbial growth so; further experiments were not performed with these surface-active compounds. The highest keratinase activity (399 U/mL) was observed in the fermentation medium containing 0.5% tween 80 followed by 0.1% tween 80 with 200 U/mL. Without surface-active compounds addition (control) into the fermentation medium, enzyme activity attained to 111 U/mL at 4th day. Keratinase activity with respect to time and the positive effect of tween 80 on the enzyme activity were shown in Figure 2. While the keratinase production in the fermentation medium containing 0.1% of Tween 80 was higher than the control, the obvious exponential increase was provided with 0.5% concentration of Tween 80.
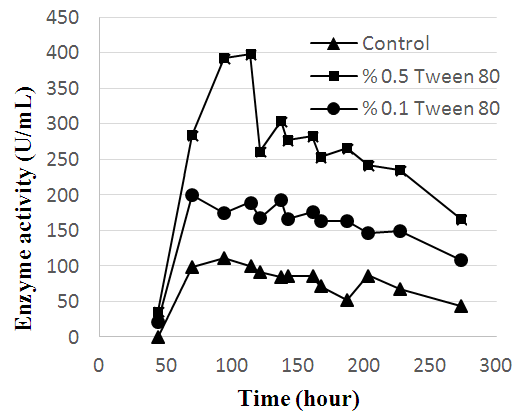 | Figure 2. Alteration of the keratinase activity according to the time |
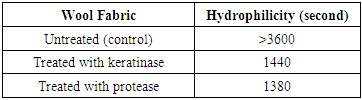
3.2. Hydrophilicity
- The effect of protease and keratinase pre-treatments on wool was shown in Table 1. Both of these treatments improved the hydrophilicity of wool fibers due to the removing of bounded fatty acids on wool surface. This treatment improves hydrophilicity of wool fibers significantly.
|
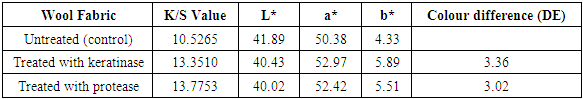
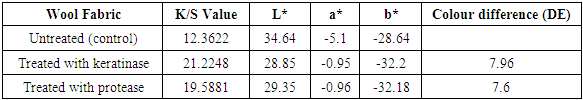
3.3. Dyeing Properties
- DE, L*, a* and b* values of untreated and enzyme treated dyed wools were given in Table 2 and 3. L* (lightness) values showed decrease of enzyme treated fabrics compared to untreated fabric. It could due to the penetration of more dyes into the enzyme treated wool fabrics, which affects the exhaustion values. Enzyme treated wool fabrics gave relatively small differences of a*, b* values. Increment in K/S values also showed the effect of cutinase and protease treatment on wool fabrics [20]. The improvement in colour strength is very significant especially for keratinase on dyed fabrics with Realan Blau RC.
|
|
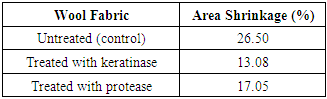
3.4. Shrinkage Properties
- Table 4 shows the area shrinkage of wool fabrics treated with enzymes. Keratinase cleaved some disulphide cross-links instead of removing some peptides from wool scales and gave shrinkage value at 13.08%. Protease treatment gave 17.05% decrease in area shrinkage values compared to the untreated fabric. Low shrinkage value means better anti shrinkage property after enzymatic treatment.
|
3.5. Surface Observation
- SEM images of untreated and treated wool fibers with keratinase and protease were given in Figure 3. The significant differences between untreated and enzyme treated fibers could be seen clearly from Fig (b) and (c). SEM micrographs also proved that the keratinase treatment gave rise to the proteolysis of cuticle layers resulting a partial removal of the scales with smooth edges. Also, there was seen no noticeable differences between keratinase and protease treated fibers [18].
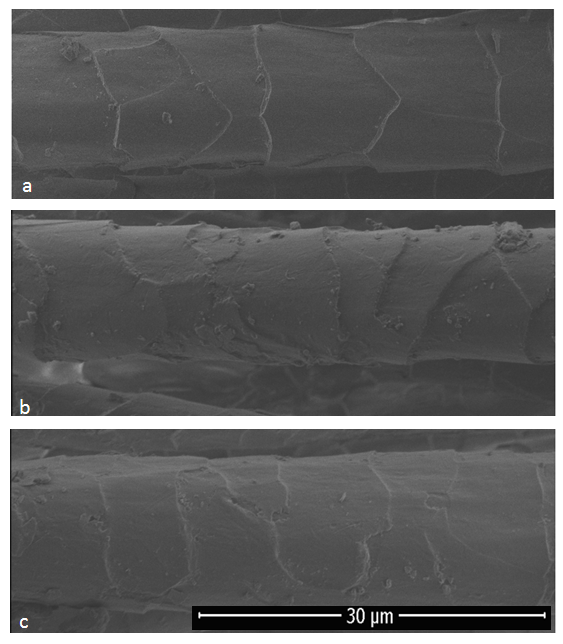 | Figure 3. SEM images of untreated and treated wool fibers a) Untreated, b) Treated with keratinase, c) Treated with protease |
4. Discussion
- The demand of keratinolytic proteases is still increasing due to its potential usage in different industries. Textile sector is the main industry using this enzyme especially for the processing of wool fabrics. The surface of wool consisting epicuticle, exocuticle, endocuticle, has a significant role in finishing processes. Enzymatic process, as an important alternative method to chemical processes, improves shrink resistance, hydrophilicity and dyeing properties of wool fabrics without damaging the fibers. Wool fibers are strongly hydrophobic due to the epicuticle and exocuticle layers of wool scales. Although wool gave satisfactory performance for fermentation process as a substrate, it is not readily available when compared to glucose. Therefore, its usage as raw substrate for fermentation process, requires additional interventions such as surface-active compounds.Streptomyces is the largest and an important genus belonging to actinomycetes in Bacteria domain. They are especially known for the capability of producing a wide variety of enzymes, so the interest of researchers for investigating these organisms is increasing with a considerable rate. There are several studies with Streptomyces species for protease production and also keratinase producer members of them have been described [6, 9]. In previous researches, keratinase production was performed by generally feather-degrading microorganisms therefore; there are very limited studies about wool-degrading microorganisms [15, 21].Keratinolytic proteases hydrolyse the cuticle of the wool and penetrate into the fibers during wool processing. After the enzymatic treatment, smaller peptide and protein segments are removed, proteolytic reaction increases and hydrophilic surface occurs. Moreover, proteases remove the scale cuticles, which have a tendency to felting shrinkage, or smoothed the edges. By this way, the shrink proofing of wool surface can be obtained. However, in very few studies, keratinases were used to improve the properties of wool fabrics in the textile industry [22]. Keratinase production with required specifications for treating wool fabrics by utilizing raw wool as a substrate was the main idea of this study. On the basis of this approach, Streptomyces sp. 2M21 was chosen as a keratinase producer and raw sheep wool was used as a nitrogen and carbon source for keratinase production. In this study, to facilitate the usage of wool by the Streptomyces sp. 2M21 during the fermentation, different surface-active compounds were added into the medium. According to the results, tween 80 increased the keratinase production to high levels comparing to the control group. The presence of tween 80 might be solved the waxy lipids on the wool surface in fermentation medium and thus improve the productivity by facilitation of the degradation process. The application potential of keratinase showed similar results in terms of hydrophilicity, dyeing and shrinkage properties of wool fabrics compared to commercial protease enzyme at lower temperatures.
5. Conclusions
- It has been determined that in the production of keratinase from Streptomyces sp. 2M21, sheep wool can be used as substrate and the production efficiency can be increased by using surface active compounds. Sheep wool residue can be evaluated as a substrate in the production process of this enzyme, which has been shown to have potential for use in textile finishing at low temperatures.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML