-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Textile Science
p-ISSN: 2325-0119 e-ISSN: 2325-0100
2018; 7(2): 35-42
doi:10.5923/j.textile.20180702.01

Ecofriendly Sweet Scented Osmanthus Leaf Extract Mediated Synthesis of Silver Nanoparticles (SNPs)
Adindu Chisom Chinyerenwa 1, Md Kamrul Hasan Munna 1, Saifur Rahman 1, Md Rasel Mia 1, Md Abu Yousuf 1, Jafrul Hasan 2
1School of Textile Science and Engineering, Wuhan Textile University, Wuhan, 430200, China
2BGMEA University of Fashion & Technology
Correspondence to: Adindu Chisom Chinyerenwa , School of Textile Science and Engineering, Wuhan Textile University, Wuhan, 430200, China.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

A non-toxic, ecofriendly method was used to synthesize colloidal solutions of silver nanoparticles in this study by using Sweet scented osmanthus leaf extract as the reducing and stabilizing agent at different temperatures (25°C, 40°C and 60°C) for the first time, hence, there was no need for toxic and costly reducing and capping agents, thus no chemical was used in the synthesis. The formation of silver nanoparticles was monitored by UV–Vis spectrophotometer analysis. The synthesized silver nanoparticles were characterized by; X ray Diffraction (XRD), Fourier Transfer Infrared Spectroscopy (FTIR), Scanning Electron Microscope (SEM), Thermo gravimetric analyzer (TGA) and Zetasizer Nano. The characterization shows that the synthesized nanoparticles were spherical in shape and have a size range between 13.54nm to 18.17nm. It also confirmed that the silver nanoparticles have a face cubic crystal (FCC) structure and were capped by biomolecules from osmanthus leaf extract.
Keywords: Sweet scented osmanthus, Eco-friendly synthesis, Silver nanoparticles, UV-Vis spectrophotometer, Leaf extract
Cite this paper: Adindu Chisom Chinyerenwa , Md Kamrul Hasan Munna , Saifur Rahman , Md Rasel Mia , Md Abu Yousuf , Jafrul Hasan , Ecofriendly Sweet Scented Osmanthus Leaf Extract Mediated Synthesis of Silver Nanoparticles (SNPs), International Journal of Textile Science, Vol. 7 No. 2, 2018, pp. 35-42. doi: 10.5923/j.textile.20180702.01.
Article Outline
1. Introduction
- Nanotechnology is a very vital field of science. It focuses on the formation, techniques, properties and control of the structures of nano-materials. Nano-materials are materials whose dimensions are in nano-meter range. They have unique properties which have made them very important and useful in diverse areas which include; food packaging, water treatment, drug delivery, cosmetics, immunology, sensors, data storage, textiles, imaging, bio-medicals, environmental remediation, medicals, electronic, transistors, energy science, oil and gas, etc. Nanoparticles have attracted many scientist and engineers as a result of their unique properties which has facilitated their applications in diverse fields such as; water treatment and purification, drug delivery, imaging, sensors, data storage, textiles, food packaging, chemical industries, catalysis, mechanics, space industries, energy science, light emitters, nonlinear optical devices, sensors, optics, etc. [1-5].Nanoparticles can be synthesized by using the top to down approach or the bottom to top approach. Top to bottom approach involves using bulk materials of the precursor as the starting material for the formation of nanoparticles by breaking them into smaller pieces with the help of chemical, mechanical or other forms of energy, laser ablation sputtering, etc. The bottom to top approach involves the synthesis of nanoparticles from atomic or molecular species of the precursor by using chemicals or bio-chemicals and allowing the particles of the precursor to grow in size [6, 7].Bio-synthesis of nanoparticles involves the use of plant extracts, fungi, microbes, bio-generated polymers like sodium alignate, starch, glucose, etc. Many metal nanoparticles have been synthesized using bio-synthesis technique examples are; iron, copper, zinc, titanium dioxide, gold, silver, etc. [8-10]. Sweet scented osmanthus is (Osmanthus fragrans) is a vital ornamental plant. It is an evergreen green tree. It belongs to the family of Oleaceae. Sweet osmanthus flowers are used as natural and functional food flavour additives. Furthermore, they also have prospective medicinal value as a result of their good scent and biological properties. It contains anti-oxidants, flavonoids, melanin, phytochemicals, volatile compounds, aroma active compounds, etc. The flowers are employed in making, food, beverages, teas, etc. The volatile compounds of Osmanthus include; trans-linalool oxide, trans-β-ocimene, trans-β-Ionone, linalool, dihydro-β-ionone, β-myrcene, γ-decalactone, cis-linalool oxide, etc. Aroma active compounds found in osnmanthus are; D-limonene, hexyl butanoate, cis-β-ocimene, trans-β-ocimene, cis-3-hexenyl butanoate, trans-linalool oxide, linalool, cis-linalol oxide (pyranoid), allo-ocimene, neo-allo-ocimene, α-ionone, trans-β-ionone, cis-linalool oxide, etc [11-16].Silver nanoparticles (SNPs) have attracted the interest of many scientists and engineers as a result of their unique properties such as; antimicrobial, outstanding plasmonic property, large surface area to volume ratio, electromagnetic, chemical stability, optical, antifungal, antiviral agent, anticancer agent, good conductivity, catalytic, anti-proliferative and anti-inflammatory properties. They can be synthesized via; chemical, physical or biosynthesis method. Radiolysis, electrochemical techniques, physicochemical reduction and chemical reduction with different inorganic and organic reducing agents are the most widely used approaches in the chemical synthesis of silver nanoparticles. Evaporation-condensation and laser ablation are the main physical techniques for making nano-particles using the physical method. [17-19]Biosynthesis method is of great importance because it is an ecofriendly method which does not need toxic chemicals hence avoids pollution. Silver nanoparticles have been produced using biosynthesis method examples include using; bacteria, fungi, microbes, plant extracts, natural polymers. [20-23].The unique properties of silver nanoparticles have made them very important and useful thus giving rise to many applications in diverse fields and areas like; health care, electronics, cosmetics, environment, health, single electron transistors, biomedical, food and feed, drug-gene delivery, chemical industries, catalysis, mechanics, bio-medicals, space industries, energy science, light emitters, mirrors, nonlinear optical devices, waste water treatment, sensors, optics, dentistry, textile industries, imaging, data storage, pharmaceuticals, photo-electrochemical applications, etc. [19, 24-28]
2. Experimental
2.1. Materials and Methods
- Silver nitrate (AgNO3) was purchased from Sinopharm Chemical Reagent Co. Ltd. Beijing China. Fresh leaves of sweet scented Osmanthus were picked from Wuhan Textile University Sunshine Campus.
2.2. Preparation of Leaf Extract
- Fresh leaves of Osmanthus picked from the tree were washed thoroughly with running tap water, then rinsed properly with double distilled water, shade dried for 10 days, grinded with a dry blender, sieved with a sieve and stored for use. 3 grams of the dry leaves was weighed and put in a beaker containing 50ml of distilled water and put in a water bath with constant magnetic stirring at 60°C for 13 minutes, then removed from the water bath allowed to cool to room temperature and filtered using Whatman no. 1 filter paper to obtain Osmanthus leaf extract.
2.3. Preparation of Silver Nanoparticles
- Figure 1 shows the flow chart for the synthesis of silver nanoparticles with Osmanthus leaf extract. 1mM of silver nitrate solution was prepared by dissolving AgNO3 in double distilled water at room temperature. 10ml of the leaf extract was added into a beaker containing 90ml of AgNO3 (1mM) and stirred constantly on a magnetic stirrer at different temperatures which are; 25°C, 40°C and 60°C for 75 minutes. The formation of pink red solution signified the formation of SNPs. UV spectrophotometer was used to monitor the formation of the silver nanoparticles (AgNPs) by periodic sampling by the synthesized NPs. The as synthesized nanoparticles were centrifuged at 10000 rpm for 20 minute then, dispersed in double distilled water and centrifuged again. The dispersion in double distilled water and centrifugation processes were repeated 5 times, so as to remove unreacted phytochemicals of the leaf extract. The SNPs were then dried at 60°C.
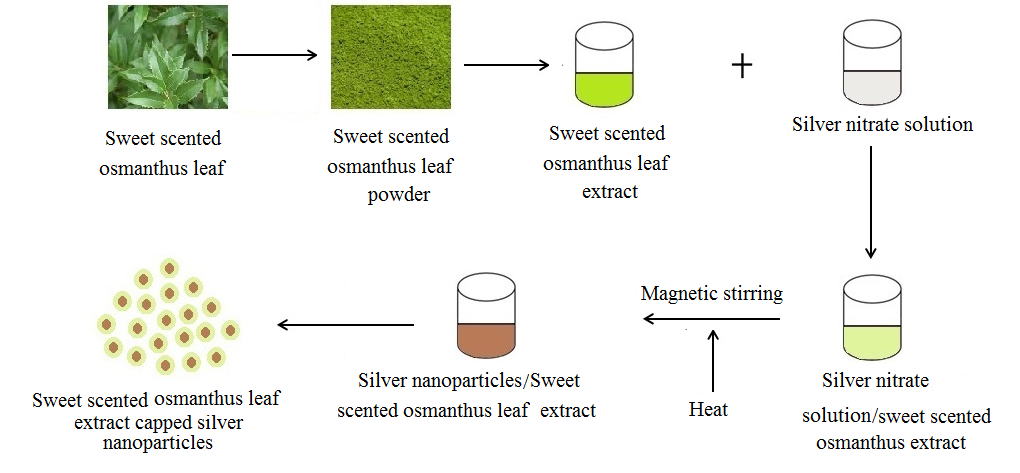 | Figure 1. Schematic diagram for the synthesis of silver nanoparticles using sweet scented osmanthus leaf extract |
2.4. Characterization
- The bio-reduction of Ag+ ions was monitored by adsorption spectra analysis of the synthesized SNPs which was done with a UV-2550 Uv-Vis Spectrophotometer Shimadzu Japan with two 10 mm optical-path-length quartz cuvettes at a resolution of 1 nm in the range of 400-550 nm so as to determine the effect of temperature and the effect of time on the synthesis of SNPs with osmanthus leaf extract as a reducing and capping agent by using two quartz cells in which one of the cells contained double distilled water as the control while the other contained the synthesized nanoparticles. Scanning Electron Microscope (SEM) (JEOL JSM-6510LV) was employed in the evaluation of the morphology of the synthesized nanoparticles. Small drops of the nanoparticles that were dispersed in water were dropped on a copper grid, dried under mercury lamp, coated with carbon and used for the morphological analysis. The study was carried out at an accelerating voltage of 20kV. The functional groups responsible for the bio-reduction were evaluated using Fourier Transform Infrared (FT-IR) Bruker Tensor 27 Germany with OPUS software. The analysis was done in the range of 400-4000cm-1 by using potassium bromide (KBr) so as to analyze the functional groups present in the as synthesized NPs. The thermal stability of the prepared SNPs was studied with SDT Q 600 Thermo Gravimetric Analyzer (TGA) (T.A. Instruments-water LLC, Newcastle, DE, USA) from room temperature to 1000°C at a heating rate of 20°C/min under nitrogen atmosphere. XRD patterns of our silver nanoparticles were recorded by using X`Pert PRO X-ray diffractometer so as to study the crystslinity of the synthesized AgNPs. The analysis was carried out at 40 kV and 40mA at room temperature in 2 thetao range from 2 theta = 20o to 85o. Zetasizer Nano is used to measure the size, molecular weight, and zeta potential of particles and molecules dispersed in solution. Zetasizer Nano 3600 was used in this study to appraise and record the size of the synthesized nanoparticles.
3. Result and Discussion
- The formation of silver nanoparticles in this study was confirmed by UV–visible spectral analysis.
3.1. Optical Evaluation
- The reddish brown colloidal suspension of the reaction mixture of osmanthus leaf extract and AgNO3 was used as a visual check for the formation of Ag NPs. The formation of silver nanoparticles was also confirmed using UV–Visible spectroscopy due to Surface Plasmon Resonance phenomenon. The study was carried out at constant time intervals so as to evaluate the effect of time on the synthesis of the SNPs as time is a very crucial factor in the synthesis of silver nanoparticles. Silver nitrate solution was reduced to silver nanoparticles in aqueous media by aqueous sweet scented osmanthus leaf extract. The UV-Vis spectra the synthesized nanoparticles show the absorbance peaks of the AgNPs at 382nm and 420nm which is as a result of surface plasmon resonance. It has been observed that silver nanoparticles show plasmon resonance absorbance in the wavelength range between 350nm to 690nm depending on the size of the nanoparticles [29-31]. The fact that our nanoparticles showed maximum absorbance at 380nm means that they are small sized nanoparticles. The absorbance of the nanoparticles increased with increase in time as shown in figure 2. This phenomenon can be because more nanoparticles were synthesized as time increased from 15 to 75 minutes. While the effect of temperature shows that the amount of SNPs synthesized increased with increase in temperature as was also observed visually that the color became darker (intense) with increase in temperature as seen in figure 3.
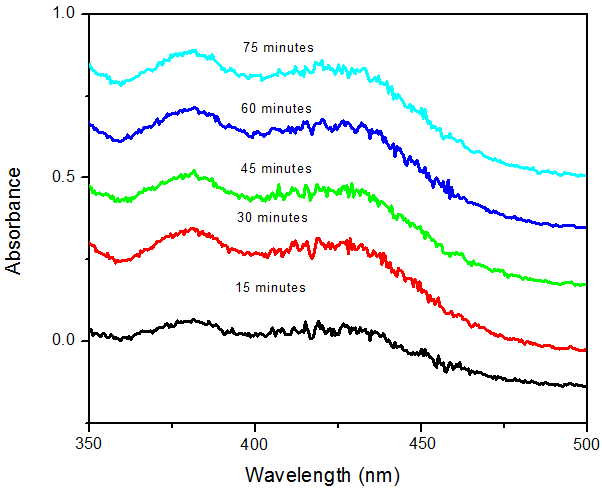 | Figure 2. The formation of silver nanoparticles with AgNO3 and Osmanthus fragrance leaf extract |
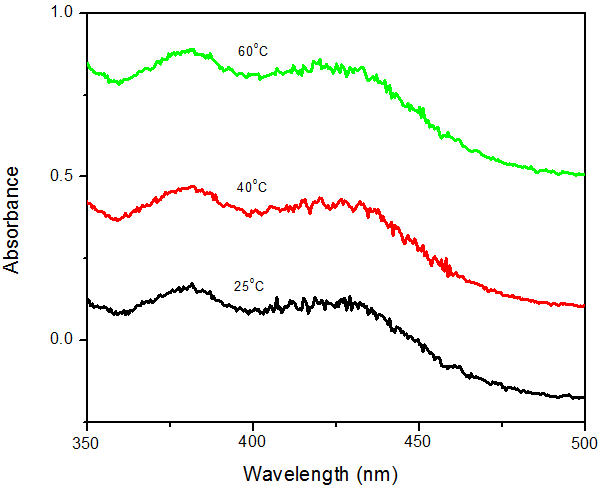 | Figure 3. Effect of temperature on the synthesis of silver nanoparticles with Osmanthus fragrance leaf extracts |
3.2. Morhological Analysis
- The analysis of the Scanning Electron Microscopy (SEM) images evaluates the morphology of the synthesized silver nanoparticles. The SEM images in figures 4A and 4B show that the AgNPs are spherical in shape and were in clusters. They are uniform in their spherical shape.
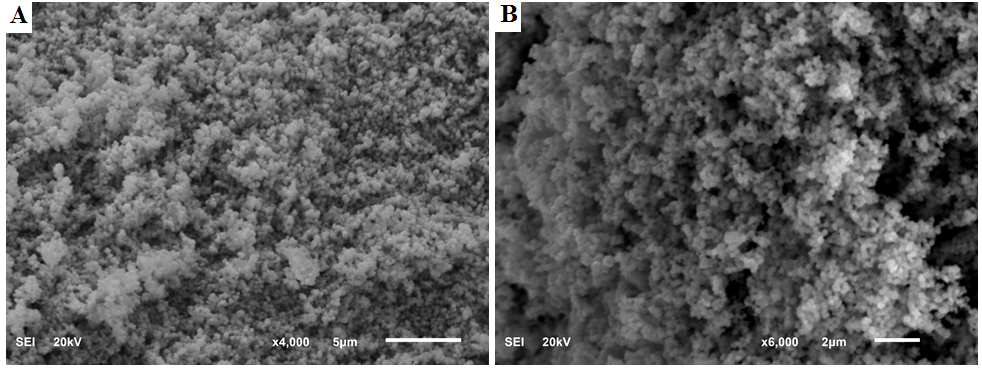 | Figure 4. SEM image of silver nanoparticles different magnifications (A) x4000 and (B) x6000 |
3.3. Chemical Characterization
- FTIR spectroscopy was used to ascertain the biomolecules and functional groups that are responsible for the reduction and capping of the as formed AgNPs. FTIR spectra of the leaf extract in figure 5A demonstrate the existence of numerous functional groups. The peak at 3414cm-1 shows OH stretch, the peaks at 2924cm-1 and 2855cm-1 designates methylene C-H asymmetric and symmetric stretch respectively, the peak at 1729cm-1 reveals the presence of ketone or carboxylic acid, the peak at 1650cm-1 shows alkenyl C=C stretch, 1514cm-1 confirms C=C-C aromatic ring stretch, the peak at 1456cm-1 indicates to methylene C-H bend, 1270cm-1 and 1320cm-1 are showing OH in plane, 1040cm-1 and 1155cm-1 are evidence of skeletal C-C vibrations, 775cm-1 and 825cm-1 are indication of aromatic C-H out of plane bend, 667cm-1 denotes alkyne C-H bend and 603cm-1 suggests S-S stretch. Some of these functional groups are responsible for the reduction of silver salt to silver nanoparticles as illustrated in the FTIR spectra of our silver nanoparticles in figure 5B. The peak at 3378cm-1 represents OH stretch, 2926cm-1 connotes methylene C-H asymmetric stretch, 1714cm-1 indicates ketone or carboxylic acid, 1629cm-1 implies alkenyl C=C stretch, 1521cm-1 denotes C=C-C aromatic ring stretch, 1384cm-1 designates C-H group, 1270cm-1 is illustrating OH in plane bend, 1076cm-1 and 1040cm-1 are proof of skeletal C-C vibrations and 670cm-1 shows alkyne C-H bend.
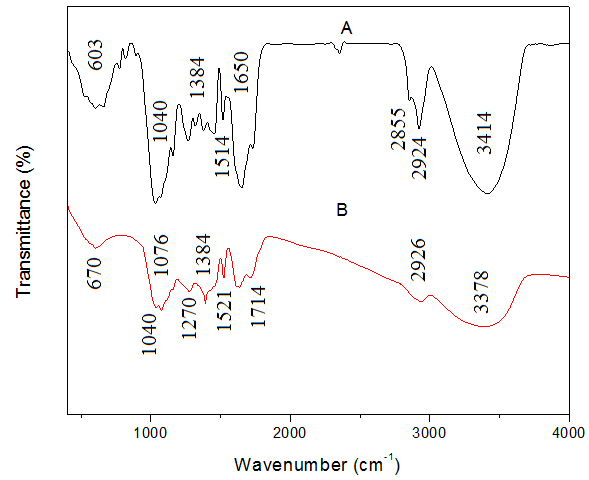 | Figure 5. FTIR spectra of (A) and sweet scented osmanthus leaf extract (B) Synthesized AgNPs |
3.4. Thermal Analysis
- The thermal properties of the nanoparticles were studied via TG analysis and showed 3 stages of weight loss. The first stage of weight loss took place between 50°C to 173°C. This loss could be attributed to loss of bound water molecules (evaporation of water molecules) on the nanoparticles. The second stage of weight loss took place between 173°C to 745°C. This weight loss could be attributed to the decomposition of the phytochemicals of the leaf extract capping the nanoparticles. The last stage of weight loss took place between 745°C to 1000°C and may be due to the degradation of the AgNPs. These weight losses are illustrated in figure 6.
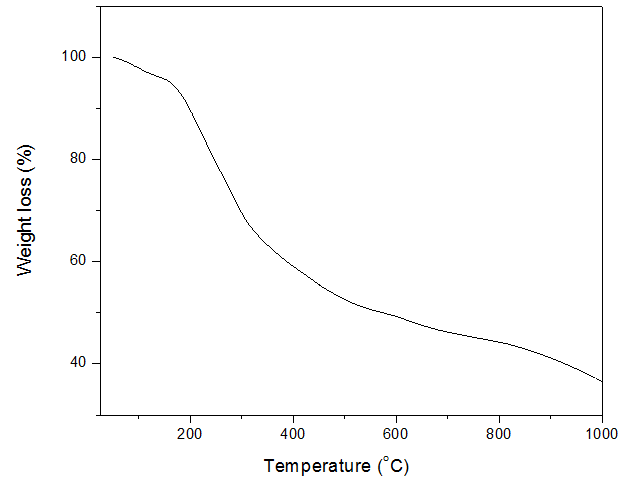 | Figure 6. TG curve of the synthesized AgNPs |
3.5. Structural Characterization
- X-ray diffraction analysis was done so as to find out the phase and crystallinity of the synthesized NPs. The X-ray diffraction pattern of the AgNPs synthesized as can be seen in figure 7 has four peaks at 38.14°, 44.41°, 64.57°, 77.42° and 82.58° corresponding to the (111), (200), (220), (311) and (222) planes confirmed the formation of AgNPs as all the peaks corresponded to the face centered cubic symmetry of silver nanoparticles [32-35]. The diffraction peaks data obtained were in accordance with the reports of FCC structure from Joint Committee Powder Diffraction Standards (JCPDS) file No. 04–0783. The intense peak at 38.14° shows the high degree of crystalinity of the synthesized AgNPs. There is no peak of impurity which means that the synthesized silver nanoparticle is purely silver nanoparticles without any impurities.
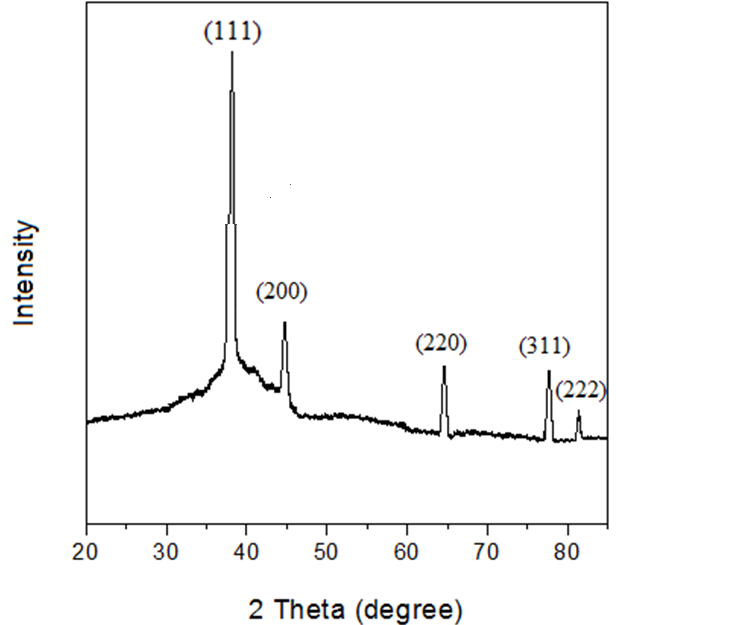 | Figure 7. XRD curve of AgNPs |
3.6. Size Distribution of the Silver Nanoparticles
- The zetasizer nano 3600 was used to study the result of the synthesized nanoparticles and the result shows that the nanoparticles synthesized have a size range between 13.54nm to 18.17nm. This is shown in figure 8.
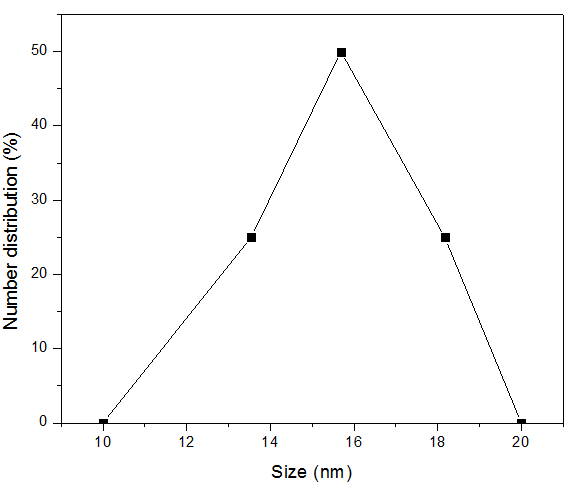 | Figure 8. Number distribution and size (nm) of the AgNPs |
4. Conclusions
- Silver nanoparticles have been successfully synthesized via a green synthesis method with osmanthus leaf extract. The synthesized nanoparticles have a size range between 13.54nm to 18.17nm. XRD and UV-Vis analysis confirmed the formation of silver the nanoparticles. The amount of silver nanoparticles formed increased with increase in reaction time and increase in temperature. The synthesized nanoparticles have potential application in waste water treatment, bio-medicals, medical textiles, wound dressing, antimicrobial applications, etc. The simple procedure used in the synthesis of the silver nanoparticles in this study has the advantage of large scale production as it can be used efficiently in large scale production.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML