-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Textile Science
p-ISSN: 2325-0119 e-ISSN: 2325-0100
2017; 6(6): 153-157
doi:10.5923/j.textile.20170606.03

Impact Analysis of Electro Spun Nano Fiber from Biodegradable Polymer for Tissue Engineering- A Review Article
Md. Rasel 1, Sojib Raihan 1, Israt Zerin 2, Mohammad Tofayel Ahmed 1, Md. Shah Alam 1, Habibur Rahman Abir 1
1Department of Textile Engineering, Southeast University, Dhaka, Bangladesh
2Department of Yarn Engineering, Bangladesh University of Textiles, Dhaka, Bangladesh
Correspondence to: Md. Rasel , Department of Textile Engineering, Southeast University, Dhaka, Bangladesh.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Every country in the world is now developing their medical innovation from the problems they are facing. Promising bio-materials as scaffold production from bio polymers is one of great innovation in the field of tissue engineering which has been involved to various application in the field of bio-medical engineering. There are many biopolymer can be used to produce scaffold but we have chosen PCL (POLY CAPRALACTONE) biodegradable Polymer to produce nano fibers or scaffold via electro spinning technique because it’s a easiest way for production nano fibers as scaffold. The target of this study is production of scaffold from biodegradable polymer (PCL) for tissue engineering purpose & this work is includes: - Nano fibers as scaffold production from PCL (POLYCAPROLACTONE) polymers via electrospinnig process. -Observation of the effect of solution viscosity. -Observation of solvents effect on fibers surfaces as scaffold. -Analysis surface or fibers morphology & diameter via microscopic tester.
Keywords: Electro spinning, Scaffold, Biodegradable polymer, Nano fiber
Cite this paper: Md. Rasel , Sojib Raihan , Israt Zerin , Mohammad Tofayel Ahmed , Md. Shah Alam , Habibur Rahman Abir , Impact Analysis of Electro Spun Nano Fiber from Biodegradable Polymer for Tissue Engineering- A Review Article, International Journal of Textile Science, Vol. 6 No. 6, 2017, pp. 153-157. doi: 10.5923/j.textile.20170606.03.
Article Outline
1. Introduction
- PCL is a biodegradable polymer with 59-64° temperature, Tg -60° & rubbery state [1-3]. The erosion rate of biodegradable Nano fibers are following the order PGA, PLGA PLLA, PCL [4]. Electros pining is easiest and popular technique and Electro spun Nano fibers are produced from different polymers (natural or synthetic polymers) for different applications [5, 6]. PCL is most promising synthetic/ semicrystaline biodegradable polymer & it degrades very slower [7, 8] and its with a wide range of applications in the field of tissue engineering such as delivery device or commercial sutures, biomedical materials due to physical properties [9-13] & biological properties [14-17] like blood vessel [18-20], bone scaffold [9, 21-24], nonwoven membranes [9, 25] etc. PCL has less mechanical properties but it can be blending with natural polymers [26], synthetic polymers [27, 28] by electro spinning technique for tissue engineering.
2. Raw Materials
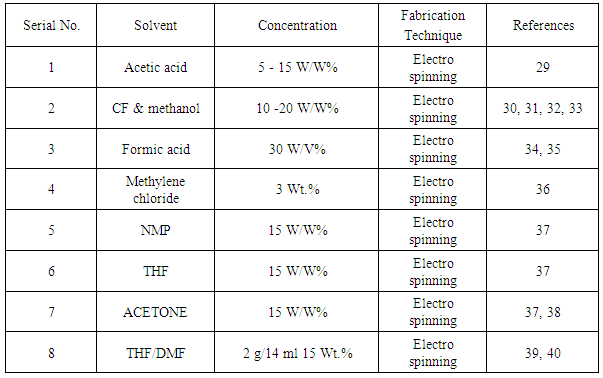
- Electro spun PCL Nano fibers which have dissolved into most of solvent but some are given below in a table:-
|
2.1. Parameters of Electro Spinning
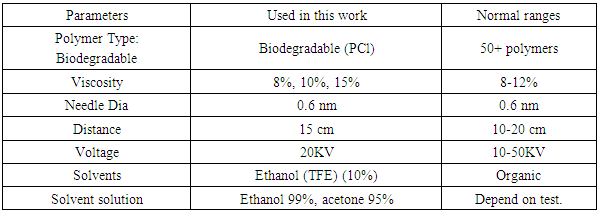
- There are many parameters can be effected on the electro spinning process such as Viscosity, conductivity, solvent, Surface tension, flow rate, collector take up, Velocity, polymer, temperature, collector, needle design, needle dia etc. Several parameters are used in this work, which are most important for electro spinning these are:-
|
3. Methodology
- PCL/ethanol & PCL acetone solution viscosity a) (PCL 3g + METHANOL 27gm) = 10% viscosity Similarly, acetoneb) 8% & 15% viscosityThese have been used to produce Nano fibers by electrospining technique.
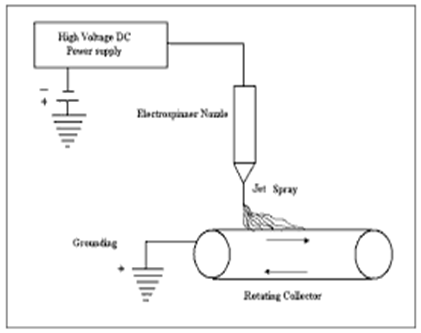 | Figure 1. Schematic representation of the electrostatic fiber spinner |
3.1. Flow Rate
- Only 10% viscosity has been showed by this figure 2.
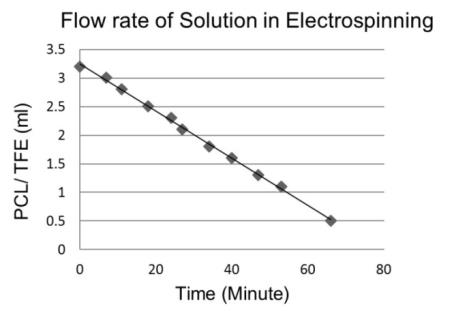 | Figure 2. Flow rate of solution |
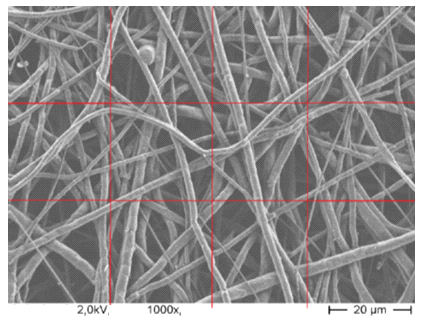
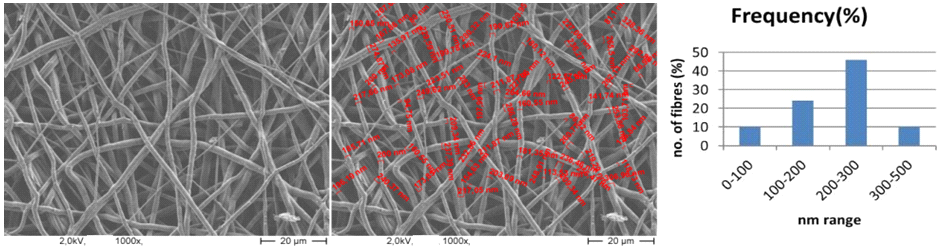 | Figure 4. The SEM image has been showed that fiber distribution is most important for cell adhesions in the scaffold as tissue engineering in human body & diameters of Nanofibers 100-500nm |
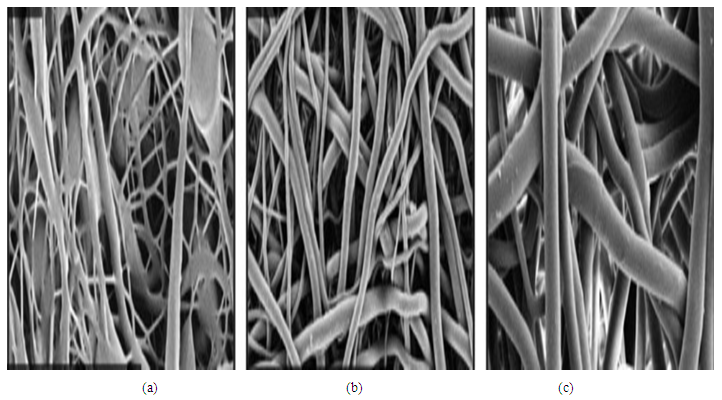 | Figure 5. Representative images of fibers spun at (a) low, (b) intermediate, and (c) high viscosity |
4. Results & Discussions
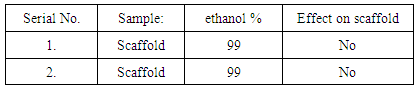
- In this work solvents taken ethanol & acetone. Scaffold samples have been produced through acetone 95% & ethanol 99%. These solvent should not modify the fibers.
|
|
5. Conclusions
- PCL is versatile polymer for tissue engineering applications. Ethanol/acetone has been used to fabricate Nano fibers as scaffold by electro spinning but they have not effect on scaffold morphology or fiber surfaces but solvents has effect on viscosity of polymer solution which can produces coarser or finer fibers diameter.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML