-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Textile Science
p-ISSN: 2325-0119 e-ISSN: 2325-0100
2017; 6(3): 79-87
doi:10.5923/j.textile.20170603.01

Synthesis, Application of a Novel Azo Dye and Its Inclusion Complex with Beta-cyclodextrin onto Polyester Fabric
H. M. Dardeer1, El-sisi A. A.2, Emam A. A.2, Nora M. Hilal2
1Chemistry Department, Faculty of Science, South Valley University, Qena, Egypt
2Department of Chemistry, Faculty of Science, Al-Azhar University (Girls), Nasr City, Cairo, Egypt
Correspondence to: H. M. Dardeer, Chemistry Department, Faculty of Science, South Valley University, Qena, Egypt.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In this study the synthesis of azo dye 4-(2-(10-oxoanthracen-9(10H)-ylidene)hydrazinyl)-N-(pyrimidin-2-yl) benzenesulfonamide (dye1) and its inclusion complex with β-yclodextrin (dye2) and their application on polyester fabric are described. The chemical structure of the azo dye formed and the inclusion dye complex created were confirmed by FT-IR, 1H-NMR and mass spectral studies. Spectral analysis reveals that the physical and chemical properties of the formed inclusion complex (dye2) are different from the azo dye (dye1). Dyeing exhaustion % of the two synthesized dyes onto polyester fabric in the temperature range between 70 and 100ºC was evaluated to compare the results. Higher temperature resulted in an increase in dyes exhaustion onto polyester- fabric; hence, the dyes adsorption is an endothermic process. The percentage rate of fixation at equilibrium time of 120 min for dye2 is much higher than that of dye1 due to the ability of cyclodextrin inclusion dye complex (dye2) to act as leveling agents achieving uniform dyeing by slowing down the movement of the dye molecules from liquid phase to the solid surface of the fabric substrate. Moreover the diffusion coefficients and activation energies of diffusion were also evaluated and discussed.
Keywords: Azo dyes, Polyester fabric, β-cyclodextrin, Inclusion complex, Exhaustion%, Fixation%, Dye quality%, Diffusion coefficients, Activation energy
Cite this paper: H. M. Dardeer, El-sisi A. A., Emam A. A., Nora M. Hilal, Synthesis, Application of a Novel Azo Dye and Its Inclusion Complex with Beta-cyclodextrin onto Polyester Fabric, International Journal of Textile Science, Vol. 6 No. 3, 2017, pp. 79-87. doi: 10.5923/j.textile.20170603.01.
Article Outline
1. Introduction
- Azo compounds are a type of chemical compounds that are continuously receiving attention in scientific research [1, 2]. They are usually strongly coloured compounds which can be intensely yellow, red, orange, blue or even green, depending on the exact structure of the molecule. As a result of their colour, azo compounds had great importance as dyes and also as pigments for a long time [3]. In fact, most of the dyes in industrial use today are azo dyes, which are mostly prepared from diazotization-coupling reaction [4, 5]. The structural features in organic compounds, that commonly produce colour [6] are > C = C <, –N = O, –N=N–, aromatic rings, > C = O and –NO2. Cyclodextrins are natural compounds which have distinct structures and ability to give inclusion complexes with different molecules through host–guest interaction [7]. Physical and chemical properties of guest molecules can be developed after forming the inclusion complex. On the other hand cyclodextrins have numerous uses in textile dyeing and washing methods as leveling agents [8] to reduce the non fixed absorbed materials from fiber by creating inclusion complex with these undesired substances. Also, cyclodextrins inclusion dye complexes can be used to remove unpleasant smells, and act as antimicrobial agent [9, 10] towards textile fibers, as a result cyclodextrins used in industrial medical textiles. Furthermore, using cyclodextrin inclusion dye complexes for dyeing purposes do not cause any environmental problems because their waste water is not toxic [11, 12]. Polyester fiber has a highly compact and crystalline structure, and is markedly hydrophobic. For this reason, its aqueous dyeing is carried out at high temperature and high pressure using hydrophobic dyes. Solvent-assisted and carrier dyeing has been widely studied as a mean of accelerating the dyeing rate, improving dye uptake and lowering dyeing temperature [13]. However, both solvents and carriers have serious problems, namely toxicity and unpleasant odor, poor light fastness, adverse effect on the physical and chemical properties of the fiber, high costs of waste water treatment and environmental contamination. Clearly, it seems sensible that efforts should be devoted towards the development of a new dyeing technique to accelerate dyeing rate, improve dye uptake and lower dyeing temperature in the areas of textiles. Comprehensive research efforts have been made in the area of cyclodextrins inclusion dye complexes that have been used for the dyeing of hydrophobic fabrics [14, 15] to modify and develop their surface functional properties.The present paper reports on the synthesis of an azo dye 4-(2-(10-oxoanthracen-9(10H)-ylidene)hydrazinyl)-N-(pyrimidin-2-yl)benzenesulfonamide (dye1) and its inclusion complex with β- cyclodextrin (dye2). Spectral methods were applied to demonstrate the chemical structures of the synthesized azo dyes. The azo dyes were applied on polyester fabric and their batch dyeing behavior including dye exhaustion, dye fixation, dye quality%, diffusion coefficient and energy of diffusion at various temperatures were evaluated and compared.
2. Experimental
2.1. Materials
- All reagents (Sigma Aldrich, 95%) and β-cyclodextrin (Acros Organics, 99%) were used as received without further purification. Scoured and bleached 100% polyester (0.22 dtex) fabric was supplied by El-Mahalla El-Kobra Company. The fabrics were further scoured in aqueous solution with a liquor ratio 1:50 containing 2 g/L nonionic detergent solution (Hostapal, Clariant) and 2 g/L Na2CO3 at 50°C for 30 min to remove waxes and impurities; they were then rinsed thoroughly in cold tap water, and dried at room temperature [16]. All other chemicals were purchased with high purity from Merck (Darmstadt, Germany).
2.2. Synthesis of Dyes
- 2.2.1. Synthesis of 4-(2-(10-oxoanthracen-9(10H)-ylidene)hydrazinyl)-N-(pyrimidin-2-yl) benzenesulfonamide (dye 1)Sulfadiazine (1.28 g, 5.12mmole) was dissolved in 20 ml HCl conc. and was cooled in an ice bath to -4°C. A cold solution of sodium nitrite (0.36 g) was slowly added with continuously stirring. After diazotization was completed, the diazo solution was added to anthrone (1 g, 5.15 mmole) in sodium acetate (2 g) and acetone (15 ml). The reaction mixture was stirred for 1hour; the formed precipitated was filtered, washed with water, and dried. Afterwards, it was recrystallized from ethanol to give yellow crystals in 90% yield; m.p. 282-284°C. FT-IR (KBr, cm-1) showed the presence of broad bands due to (νNH) at 3244.65 cm-1, (νC=O) at 1650.77cm-1 and (νN=N) at 1441.53 cm-1; 1H-NMR (400MHz/DMSO) indicated at δ7.03-8.56 (m, 15H, arom. H); 11.22 (s, 1H, NH); 11.65 (s, 1H, NH); MS (m/z): 455, which corresponds to the molecular formula (C24H17N5O3S). 2.2.2. Synthesis of inclusion complex 4-(2-(10-oxoanthracen-9(10 H)-ylidene)hydrazinyl)-N- (pyrimidin-2-yl)benzenesulfonamide -β-cyclodextrin (dye2)Amixture of 4-(2-(10-oxoanthracen-9(10H)-ylidene)hydrazinyl)-N-(pyrimidin-2-yl)benzenesulfonamide (0.5 g, 1.1mmole) and β- cyclodextrin (2g, 1.76 mmole) was stirred in DMF for 72 hours at 50°C. The reaction mixture was poured onto water; the solid formed was then filtered off, washed with water, and dried to give inclusion dye complex (dye2) (0.94 gm, 0.59 mmole) as orange crystals; m.p. 325°C. FT-IR (KBr, cm-1) showed the presence of bands due to (νOH’s) at 3421 cm-1, (νC=O) at 1668 cm-1 and (νC-O-C) glucosydic at 1159 cm-1; 1H-NMR (400MHz/DMSO) indicated at δ2.13 (7H, H-4); 2.28 (7H, H-2); 2.74 (7H, H-5); 2.90 (7H, H-3); 3.66 (14H, H-6); 4.40 (broad s-OH,7H,7OH primary hydroxyl); 4.85 (7H, H-1); 5.56 (broad s -OH, 14H, OH secondary); 6.70-8.64 (m, 15H, arom. H); 11.34 (s, 1H, OH); MS (m/z): 1590, which corresponds to the molecular formula (C66H87N5O38S).
2.3. Characterization of Synthesized Dyes
- All melting points are measured on Griffin melting point apparatus. Chemical structure of the synthesized dyes was studied with Fourier transform infrared (FT-IR) spectroscopy. The FT-IR spectra (KBr) were recorded on a Shimadzu 408 spectrometer and carried out at the Central laboratory of South Valley University. Proton nuclear magnetic resonance (1HNMR) spectra were recorded using Bruker 400 MHz spectrometer. Chemical shifts are reported in ppm with TMS as an internal standard and are given in δ units. Mass spectroscopy (MS 5988 and AmD 402/3 mass spectrometers at ionization energy 70 Ev) was applied.
2.4. Dye Application
- Polyester fabrics were dipped in a dyebath containing the azo dye solution, which was prepared by dissolving (1.0 g of azo dye) in 5 ml of DMF in presence of dispersing agent [17]. The pH of the dye solution was adjusted to 4.0 by adding acetic acid. Fabric samples (1g) were introduced in a flask containing the dye bath of 2% of dye over 120 min interval at 70, 80, 90, and 100°C with 1:50 liquor ratio. The residual dye bath concentrations were measured with an UV-vis spectrophotometer (Perkin Elmer Lambda 35, USA) at λmax 439 nm for dye1 (a) and 435nm for dye 2 (b) as it appears from their absorption spectra in figure 1.
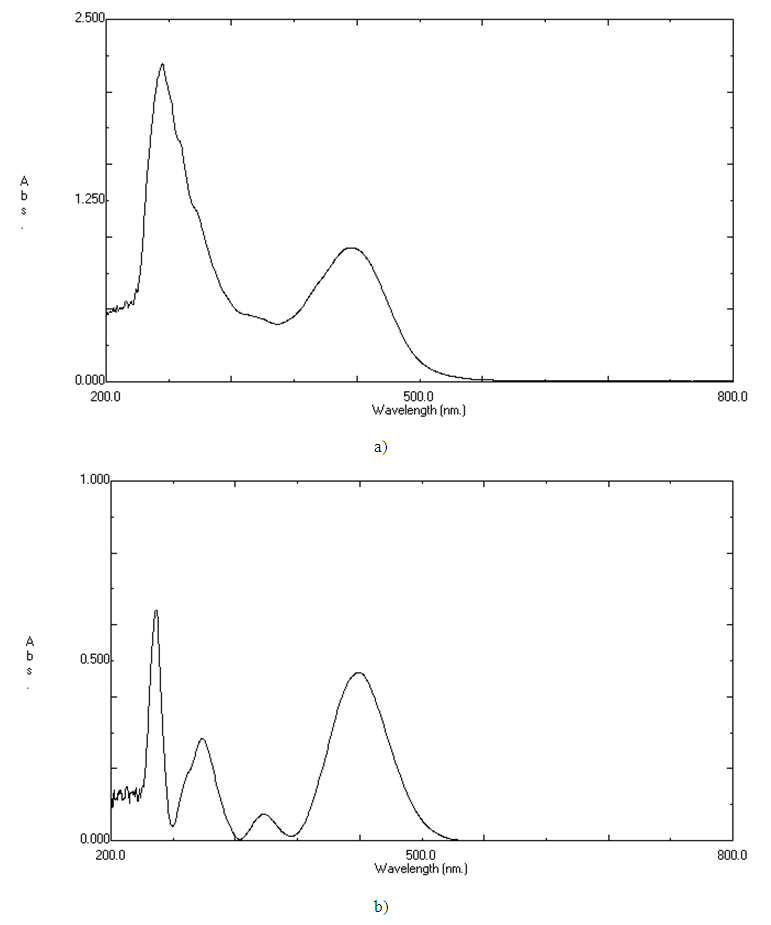 | Figure 1. Absorption spectra of a) dye 1 and b) dye2 |
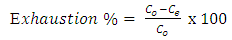 | (1) |
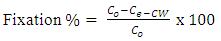 | (2) |
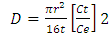 | (3) |
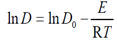 E is the activation energy (kJ mol-1); R is a gas constant (8.314 J mol-1 K-1 ) and T is the absolute temperature (K).
E is the activation energy (kJ mol-1); R is a gas constant (8.314 J mol-1 K-1 ) and T is the absolute temperature (K).3. Results and Discussions
3.1. The Fourier Transform-infrared (FTIR)
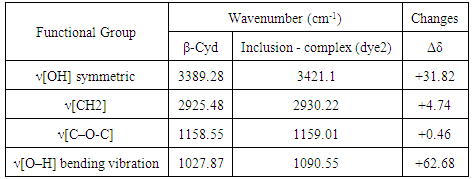
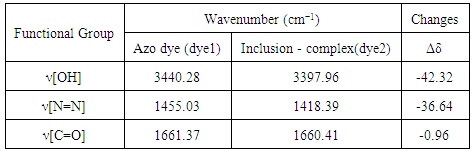
- FT-IR spectra are important technique to prove the formation of inclusion dye complex as a result of the change in frequency of functional groups of azo dye (dye1) than that of the pure β-cyclodextrin after formation of inclusion dye complex (dye 2). Figure 2 shows the FT-IR spectra for pure β-cyclodextrin (A), inclusion dye complex (B) and azo dye (C). It's observed that the spectrum of the inclusion dye complex and the spectrum of pure β-cyclodextrin are different, and this is attributed to the formation of the inclusion complex. Also, the spectrum of inclusion dye complex indicates that hydroxyl groups appear narrower than the hydroxyl groups of pure β-cyclodextrin as a result of the formation of the inclusion dye complex.Tables 1 and 2 show that there are slight increase and decrease in intensity changes Δδ. Enhancement of Δδ because of locating of the azo dye through the electron rich cavity of β-cyclodextrin which lead to increasing the frequency [7]. Also, the decreasing of intensity changes Δδ due to creation of van der Waals forces and hydrogen bonding during formation of the inclusion complex [21].
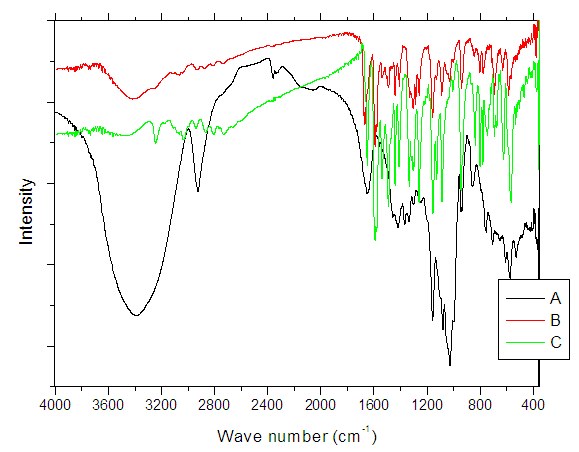 | Figure 2. FTIR spectra of β-Cyclodextrin (A), inclusion dye complex (B) and azo dye (C) |
|
|
3.2. 1H-NMR Spectra
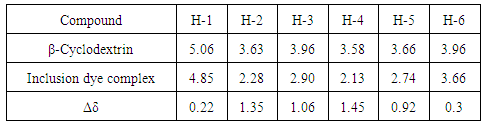
- 1H-NMR spectra for producing azo dye1 indicates the presence of aromatic protons at δ7.03-8.56 ppm and no peaks due to aliphatic protons, figure 3, also,1H-NMR spectrum of inclusion dye complex(dye2) show that appearance of β-Cyclodextrin protons in the region 2.13 to 5.56 ppm give good indication for forming of inclusion dye complex figure 4. Table 3 shows the chemical shifts observed for H-1, H-2, H-3, H-4, H-5 and H-6 for pure β-Cyclodextrin and its inclusion dye complex(dye2). Its observed that the values of chemical shift for protons of β-Cyclodextrin after formation of inclusion complex decreased and become more up field due to effect of shielding of aromatic rings for azo dye, this phenomena prove that formation of inclusion dye complex.
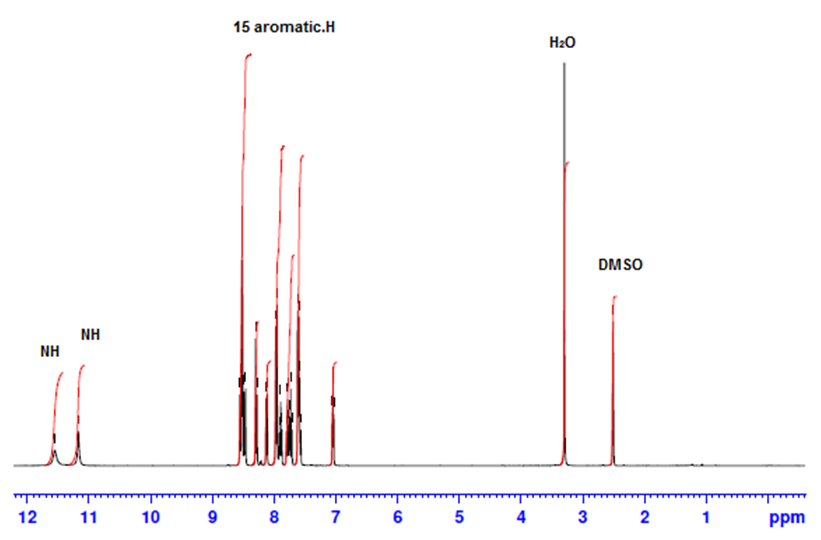 | Figure 3. 1 H NMR spectra in DMSO of (dye1) |
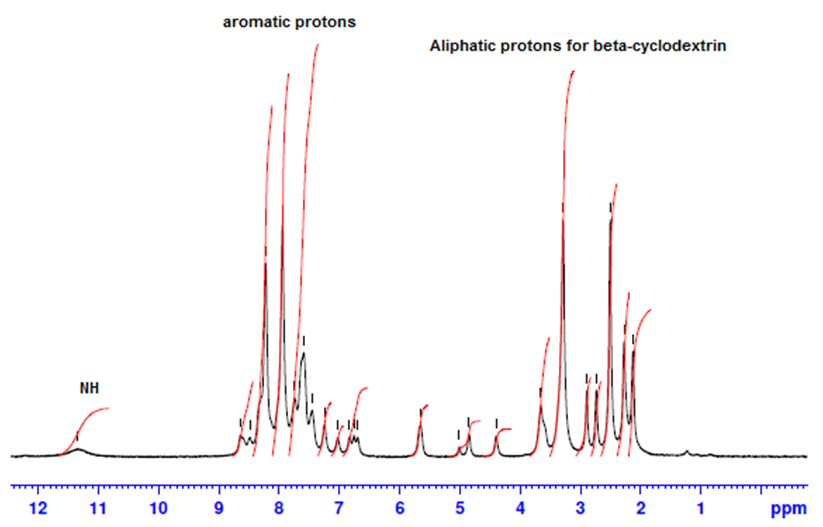 | Figure 4. 1H NMR spectra in DMSO of inclusion azo dye complex (dye2) |
|
3.3. Dyeing Kinetic Characterization
- The dyeing process is a solid/liquid phase process, which proceeds by the movement of the dye molecules from liquid phase to the solid surface of the fabric by virtue of their affinity, and then diffusion takes place inside the fabric. Therefore, the first process would be a fast adsorption controlled process where the dye molecules get into the fabric; the second slow process, which is diffusion-controlled, starts to take place. For the dye molecules to diffuse into the fabric, it is expected that the free volume could be formed within the fabric. This free volume is regarded as the void being temporarily formed within the fabric by the thermal movement of molecular chains and the dye molecules penetrate into this empty space [22]. Adsorption kinetics are important from the point of view that they control the efficiency of the process and correlate the adsorbate uptake rate with its bulk concentration. Therefore, the most practical parameters, i.e exhaustion %, fixation %, diffusion coefficient and activation energy of diffusion were determined for both types of dyes into polyester fabric.
3.4. Exhaustion %
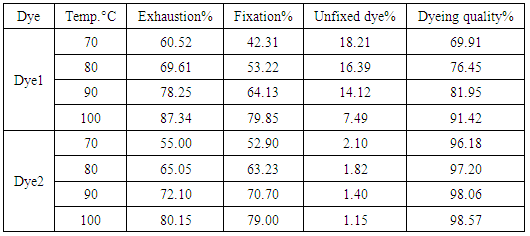
- Time-exhaustion isotherms of dyeing polyester -fabric using dye1 and dye2 at temperatures of 70, 80, 90, and 100°C over a 120 min interval are shown in Figure 5 for dye1 and figure 6 for dye2. The two figures show that the Exhaustion % (dye uptake) of polyester fabric using dye1 is generally high but not better in dyeing quality than that obtained using dye2. This is generally attributed to the fact that the β-cyclodextrin/ inclusion dye complex (dye2) with the increased molecular weight (1589 gm mol-1) does not diffuse rapidly within the fibre and has low substantivity for the textile substrate. This retarding mechanism is due to the formation of a dye/β-cyclodextrin inclusion complex (scheme 1). This complex would slow down the rapid uptake of the dye by the fibre. Agents having affinity to the dyes slow down the dyeing process by forming complexs with the dyes. The complex compound moves slower compared to the dye itself. Savarino with co-workers [23, 24]. On the other hand cyclodextrin inclusion dye complex showed a slight bathochromic shift of the absorption maxima of dye molecule. They are used as retarding agents during dyeing of the polyester fibers [25]. Higher temperature resulted in an increase in dye exhaustion onto fabric; hence, the dye adsorption is an endothermic process.
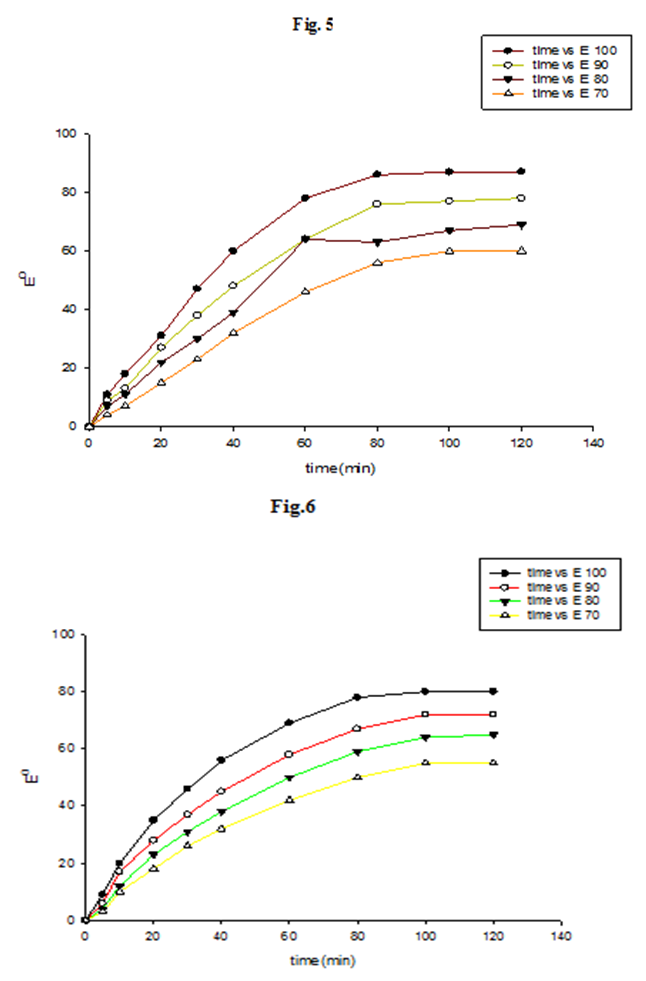 | Figure 5,6. Time-exhaustion isotherms of dyeing polyester -fabric using dye1(fig 5) and dye2(fig 6) at different temperatures [dyeing conditions; 2%owf; LR 1:50; pH=4] |
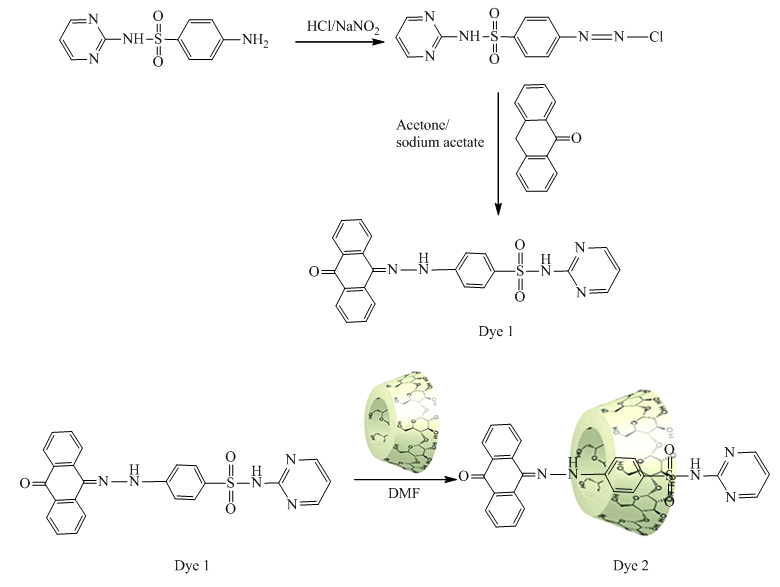 | Scheme 1 |
3.5. Fixation %
- The percentage rate of fixation of the two dyes at equilibrium time of 120 min is given in table (4). The data clarify that dye2 records high Fixation % than dye1. This is due to the ability of cyclodextrins inclusion dye complex(dye2) to act as levelling agents achieving uniform dyeing by slowing down the dye exhaustion or by dispersing the dye taken by the fibre in a uniform way [8], and thus cyclodextrin-dye solution showed an acceptable homogeneity in dyeing. Whereas with dye1 the coloration was heterogeneous although the higher recorded values of exhaustion % as seen from figure 5. The permanent fixation of cyclodextrin inclusion dye complex on polyester fabric surfaces as shown from the data in table 4 (fixation %) will result in textiles with new properties [26]. These properties enable cyclodextrins inclusion complexes to be used in food, pharmaceuticals, cosmetics, environment protection, bioconversion, packing and in the textile industry [27]. The ring structure of cyclodextrins allows them to act as hosts and form inclusion compounds with various small molecules as it occurs with dye2. This "molecular encapsulation" is already widely utilized in many industrial products, technologies, and analytical methods [28, 29]. Dyeing quality % was calculated and recorded in table 4 and the values of fixation % were significantly improved compared with exhaustion% leading to increase of dyeing quality % to 98.57% when β-cyclodextrin inclusion dye was used as a retarding reagent compared to dye1 at 100°C.
|
3.6. Diffusion Coefficient and Activation Energy of the Dyeing Process
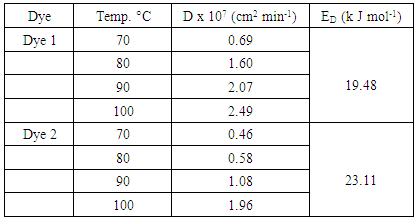
- The physical adsorption (physisorption) or chemical adsorption (chemisorption) mechanisms are often an important indicator to describe the type of interactions between dye molecules and polyester fabric. The increased diffusion coefficients of dyes with temperature rise Table (5) can be related to the polyester –dye interaction which increases the adsorption surface capacity. Lower values of diffusion Coefficient and consequently slower penetration and high dyeing quality of dye2 than dye1 were observed, which further confirm the possibilities of using cyclodextrins inclusion dye (dye2) as a dye complexing levelling agent in dyeing of polyester fabric. It also has effective influence for controlling dyeing uniformity. Furthermore, it is found that the arrangement of guest dye molecule within the cavity of the cyclodextrin host molecule gives the positive effects on the quality of polyester dyeing with dye2 than dye.The activation energy describes the dependence of the diffusion coefficient on the dyeing temperature and also represents the energy barrier that a dye molecule should have to diffuse into the fabric polymer chain [30]. The low activation energies are characteristic for a physical adsorption (5-40 kJ mol-1), while higher activation energies (40-800 kJ mol-1) suggest chemical adsorption [31]. The activation energy of the diffusion of dye1 and dye2 was calculated by Eq. 4 from the linear relationship of log D against 1/T for both dyes, Fig. 7. The diffusion coefficient and activation energy results for dyeing of polyester -fabric using both dye1 and dye2 are demonstrated in Table 5. Considering these values are in the typical activation energy range for physisorption, one can conclude that dye1 and dye2 adsorbed mainly physically. Furthermore the activation energy of diffusion of dye 2 onto polyester -fabric (23.11 kJmol-1) is significantly higher than that of dye1 (19.48 kJmol-1) their positive values confirmed again the endothermic nature of the overall dyeing processes.
|
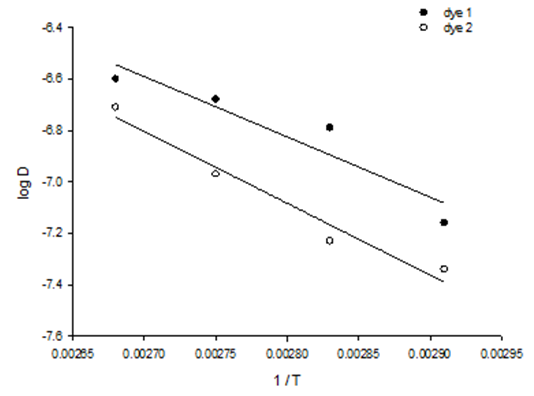 | Figure 7. Estimation of the activation energy of the diffusion of dye1 and dye2 onto polyester fabric |
4. Conclusions
- This paper describes:- The synthesis of 4-(2-(10-oxoanthracen-9(10H)-ylidene)hydrazinyl)-N-(pyrimidin-2-yl)benzenesulfonamide (dye 1) and its inclusion complex with β-cyclodextrin ( dye2). Their structures were confirmed by FT-IR, 1H-NMR and mass spectral studies.- The two azo dyes prepared in this study are exhibited good (dye1) to excellent (dye2) dyeing properties on polyester fabric.- The values of fixation % and dyeing quality % suggests that the formation of inclustion complex (dye2) via interaction of cyclodextrins host with dye guest should play a significant role in achieving uniform acceptable homogeneity dyeing by slowing down the dye exhaustion or by dispersing the dye taken by the fibre in a uniform way, whereas with dye1 the colouration was heterogeneous although the higher recorded values of exhaustion %.- Diffusion coefficients increase with temperature rise and varied from 0.69-2.49x10-7 cm2min-1 for dye1 when the temperature increased from 70 to 100°C and from 0.46-1.96x10-7 cm2min-1 for dye2 at the same temperatures range. This can be related to the nature of dye polyester-fabric interaction.- The activation energy of the diffusion of dye1 and dye2 was calculated and found to be in the typical range for physisorption. Furthermore the activation energy of diffusion of dye onto polyester -fabric (23.11 kJmol-1) is significantly higher than that of dye1 (19.48 kJmol-1), their positive values confirmed again the endothermic nature of the overall dyeing processes.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML