-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Textile Science
p-ISSN: 2325-0119 e-ISSN: 2325-0100
2017; 6(2): 21-33
doi:10.5923/j.textile.20170602.01

Salt Free Dyeing of Cotton Fiber- A Critical Review
Tania Aktek1, A. K. M. Malekul Millat2
1Textile Engineering Department, BGMEA University of Fashion & Technology, Dhaka, Bangladesh
2Knit Asia Limited, Dhaka, Bangladesh
Correspondence to: Tania Aktek, Textile Engineering Department, BGMEA University of Fashion & Technology, Dhaka, Bangladesh.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Reactive dyes, the most majestic dyes, lead the cotton dyeing industries still today as they exhibit versatile amenities such as full ranges of shade, convenient fastness properties and ease of application. Implications of these dyes demand a great deal of electrolyte to subdue the electric charge repulsion between dye and fiber. But use of high amount of electrolyte and unfixed dye contributes to environment pollution among which salt cannot be exhausted or destroyed. The usage of salt can be eliminated by either the use of cationized reactive dye or modification of cotton. Notable achievements are found for modification of cotton by treating with chitosan, cationic starch, cationic monomer, polymer and dendrimer. Recently attention has been given on preparation of cationic reactive dye to avoid using modifying agent and attained outcomes met the current market demand. This paper focuses on both approaches with highlighting the most recent trends adopted and recommends about most effective, economic and environmental benign process and also proposes future prospects in this field.
Keywords: Salt free dyeing, Cationic reactive dye, Modification, Cotton fiber, Chitosan
Cite this paper: Tania Aktek, A. K. M. Malekul Millat, Salt Free Dyeing of Cotton Fiber- A Critical Review, International Journal of Textile Science, Vol. 6 No. 2, 2017, pp. 21-33. doi: 10.5923/j.textile.20170602.01.
Article Outline
1. Introduction
- At this juncture, reactive dyes grab approximately (20-30)% arena of total dye market most of which are used for coloration of cotton fiber that meets demands of half of the world fiber consumption [1]. But reactive dyeing with cotton is considered as pollution generating process as huge amount of electrolyte estimated approximately 200000-250000 tons per year and 20-50% unfixed reactive dyes are discharged in the textile effluent in Europe [2]. During effluent treatment, a small portion of electrolytes is removed, the remaining lead to increase salinity in river, disturb marine life, create scarcity of fresh water, infertility of soil [3]. So salt-free reactive dyeing is a crying need for present world [4].To eliminate salt-usage different approaches have been adopted such as cationization of cotton with cationic agent and infliction of cationic reactive dye [5]. To incorporate cationic sites by reaction with hydroxyl groups of cellulose through esterification, etherification, grafting or crosslinking reaction is termed as cationization of cotton [6]. Basically quaternary or tertiary amino groups are embodied with cellulose to provide nucleophilic group to cellulose which show greater attraction for anionic dye resulting in formation of ionic bond without salt even alkali [6, 7].Again, growing environment concern and technical benefits claimed to manufacture less pollution generating reactive dye. For this purpose, Cibacon LS range reactive dyes are disclosed that run in either bifunctional group (bis monofluorotriazine) or trifunctional group (monochlorotriazine/bisvinyl sulphone also combination of monofluorotriazine and monochlorotriazine with vinyl sulphone) that lead to reduce salt usage. But excess reactive group ensure higher affinity for cotton slower the rates of migration of dye molecules due to higher molecular weight [8].Besides, complete elimination of salt usage is possible by incorporating tertiary amines into monocholorotrizinyl reactive dye due to increase fiber affinity by adding positive charge on dye. Moreover, Procion blue H-EG was disclosed in 1979 which contained quaternised nicotinic acid derivatives, applied for dyeing in absence of alkali [9, 10].Recently, a number of reactive dye contained cationic reactive group in their structure have been synthesized and investigated for zero salt dyeing with outstanding exhaustion, fixation and recognized as cationic reactive dye. Though a great deal of researches have been carried out on zero salt reactive dyeing, there are a few review papers and most of which focuses on only modification of cotton where use of cationic reactive dyes in this regard are not highlighted so much. This discussion focuses on available methods adopted for cationization of cotton and application process of synthesized cationic reactive dye to eliminate salt usage during reactive dyeing.
2. Progress of Salt Free Dyeing Process
- Immense researches have been practiced for aforementioned topics and effective results have been obtained among which most major advances are discussed here:
2.1. Chemical Modification of Cotton
- Purpose of chemical modification is to infix cationic sites to cellulose by low molecular weight cationic monomer and cationic polymer so that affinity of cotton is increased for anionic reactive dyes. Some of the emerging process are talked below:
2.1.1. Modification with Chitosan and Its Derivatives
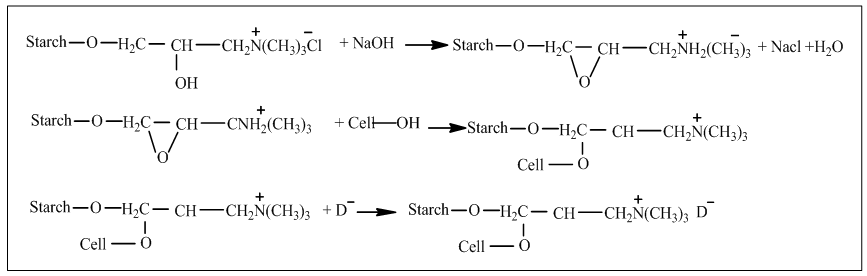
- Among of all the chemicals used for modification of cotton, chitosan is one of the safest available biocompatible material which ensures innoxiously cationization of cotton fiber in penury of salt. Actually it is a linear polysaccharide which consists of β (1, 4) linked D-glucosamine and N-acetyl-D-glucosamine which are allotted randomly in its structure. Commercially it is produced by alkaline deacetylation of chitin [6, 11]. Generally cross-linking is formed between chitosan and cotton fiber (Fig. 1) and thus introduce positive amino groups which contributes adsorption of reactive dye without salt [12]. Enormous attempts have been smeared to tone down the cotton fabric using chitosan those are described here.
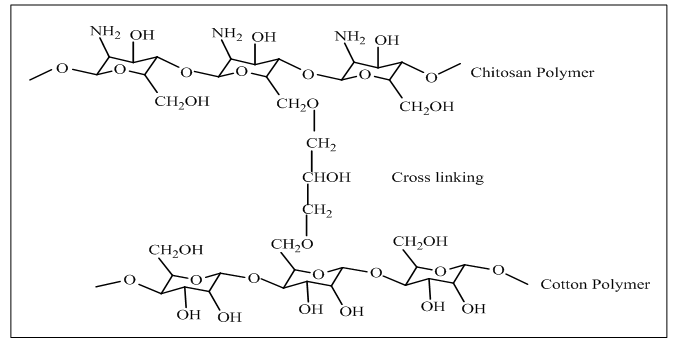 | Figure 1. Formation of cross linking of cotton polymer with chitosan [12] |
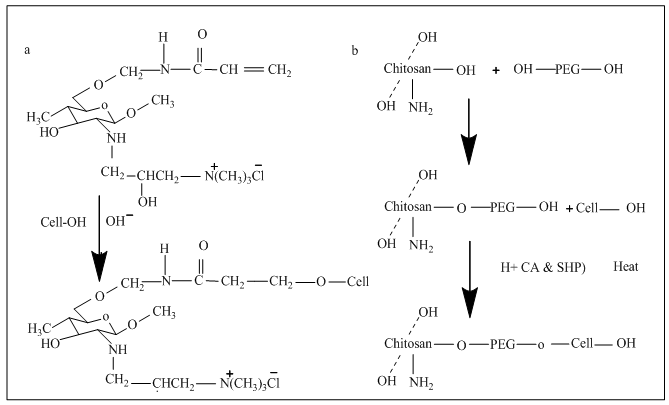 | Figure 2. a) Formation of covalent bond between cotton and water soluble chitosan [16] b) Formation of ether group between cellulose and PEG contained in chitosan [18] |
2.1.2. Modification by cationic Starch
- One of the emerging ways of physical modification of cotton is to issue cationic starch to reduce static repulsion between cotton and reactive dye which eliminate salt addition during reactive dyeing for reducing effluent discharge. Generally cationic starch is prepared from etherification of starch with ammonium cationizing reagents. Several cationic starches are synthesized recently to modify cotton some of which are mentioned here.Zhang et al., [19] reported a cationic starch which was prepared from Starch and Glytac A that was applied by pad back process (tow dips two nips pad bath and backed at 150°C for 5 min) on fabric followed by dyeing with two dips and two nips continuous process. Chemistry involved here were the essence of ionic vander walls forces and hydrogen bonding between cotton and cationic starch, also the presence of ionic bond between reactive dye and cotton. It was audited that optimal fixation of cationic starch was achieved within concentration of 0.5 to 1% and further increase in concentration would hinder the entrance of reactive dye into the cotton fiber. Again with the increase of DS, fixation level was increased and obtained dyeing properties were met the market demand.But, it was unable to apply in exhaust dyeing method in absence of levelling agent because of high viscosity. To overcome this problem, Zhang et al., [20] synthesized a series of cationic hydrolyzed starch with low viscosity and high DS from maize starch and 2, 3- epoxy propyl tri methyl ammonium chloride by dry process. Here, for preparation pad back method (padded with two dips two nips, backed at 100°C for 10 min), for dyeing exhaust method in absence of salt were used. Owing to high DS, these starches contained high positive charge which consequently helped for higher dye fixation. It was reported that initial increase in concentration leads to the increased fixation, but after certain limits further increase in concentration lowered the fixation due to removal of starch from surface of cotton during dyeing process. Besides, low viscosity benefited the penetration of dye into the fiber to obtain level dyeing by formation of ionic interaction between dye and starch. Thus ensured maximum dye utilization with low pollution from salt.Latter, Ma et al., [21] brought out a fantastic comparison on dyeing performance between cationic native starch (CNS) and cationic hydrolyzed starch (CHS) treated, untreated cotton fabric. For both CHS and CNS, dip-pad-bake (Dipped for 3 minutes, padded for 80% pick up, finally baked at 100°C for 10 minutes) method was applied for pretreatment continued dyeing in exhaust method. Dye penetration, fastness properties of CHS treated and untreated fabric were superior than that of CNS treated fabric, as higher molecular weight of CNS hindered the penetration of dye. Fig. 3 shows effect of concentration on dye fixation. From figure it was found for both CNS and CHS, initially dye fixation increased and then decreased. Optimum dye fixation 46.3% and 69.4% was achieved at 0.5% for CNS and at 2% for CHS.
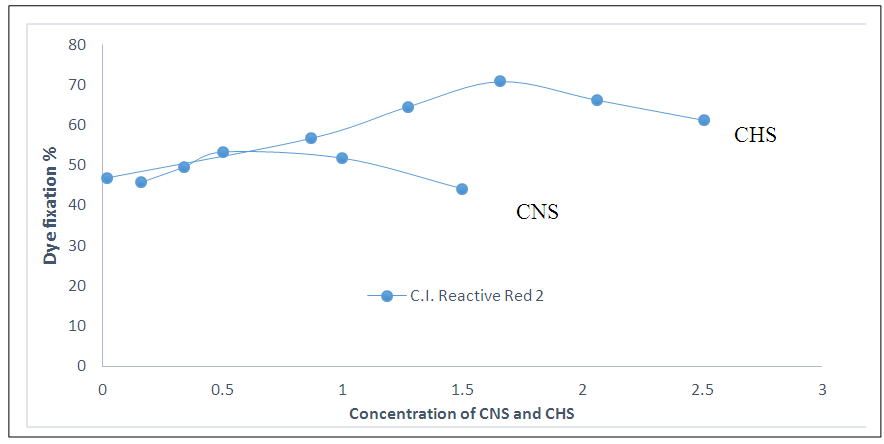 | Figure 3. Comparison of effect of concentration of CNS and CHS on dye fixation [21] |
 | Figure 4. Operation process of modifiedcotton with cationic starch [22] |
2.1.3. Modification by Dendrimer
- Dendric polymers are highly branched, spherical three dimensional structure, contain a great deal of functional endgroups which impart few special properties such as solubility, substantivity to fiber and surface activity. To permit salt-free reactive dyeing, a derivatives of Am16decanamide8 named polyamidoamine (PMMAM) (Fig. 5) was exercised on cotton fabric in exhaust method for both pretreatment and dyeing. Actually, large amount of positive charge of amino group were set up on cotton fiber during modification with PMMAM those attract anionic reactive dye as a result of which salt requirement was diminished. The result revealed that highest color strength was achieved at pH 4 in presence of levelling agent. It was also observed with theincrease of amount of dendrimer color strength increased, as increased number of amino groups were reacted with reactive dye [11, 23, 24].
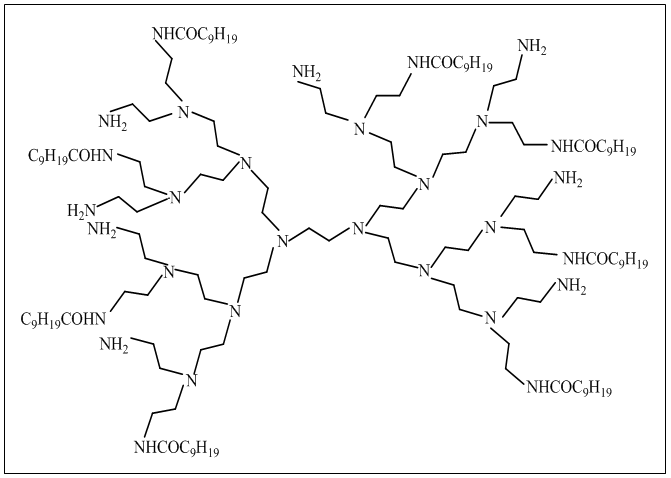 | Figure 5. Used dendrimer [23] |
2.1.4. Modification by Grafting
- Grafting of cellulose with cationic agent is opened up a new dimension to establish zero salt reactive dyeing. A few investigation have been done on grafting of cellulose those are mentioned here.Srikulkit and Larpsuriyakul [25] adopted a completely new technique, simultaneous bleaching and grafting where hydrogen per oxide was bleaching agent, methacryloylaminopropyltrimethylammonium chloride (MAPTAC) was used as grafting agent. Actually graft polymerization was attributed to convert low molecular weight cationic monomer to high molecular weight cationic polymer that could be fixed covalently with cellulose (Fig. 6a). Optimum condition for graft polymerization of cellulose was 40g/l MAPTAC and 4% o.w.m (on the weight of MAPTAC) Potassium Persulphate for 45 min at 70°C, following bleaching with hydrogen per oxide, sodium hydroxide and stabilizer. Oxidizing the hydroxyl groups of cellulose to free radical was the function of redox initiator, Potassium Persulphate. However, the obtained results suggested that whiteness index was slight low with increase of concentration of MAPTAC as residual MAPTAC solution consumed some hydrogen per oxide resulting in reduction of overall performance of bleaching. After dyeing the modified cotton, the data obtained for measurement of dyeing performance like color strength and fixation showed increased concentration of MAPTAC lead them as the more cationic groups on cotton the more anionic dyes were attracted. Here the curve obtained from adsorption of dye into the cotton fiberdid not follow the Langmuir-type adsorption curve that gave strong evidence about not only ionic bond was formed but also vander walls and hydrogen bond were formed between dye and fibers.Later, Zhang et al., [26] generated anamino-terminated hyper branched polymer grafted cotton fibre (HGCF) (Fig. 6b) from grafting of periodate oxidized cotton fiber with amino-terminated hyper branched polymer to avoid salt addition during reactive dyeing. In this process, to cleave 2, 3-vicinal diol of polymer of α-D glucose units, cotton fibres was oxidized with 2 g/l sodium periodate at 40°C for 10-180 min in exhaust method continuing the grafting of fabric with 10g/l HBP-NH2 at 60°C for 5 min, the obtained optimum condition. Thus HGCF was produced by reaction of aldehyde groups of oxidized cotton with amino groups of HBP-NH2.Then it was dyed with reactive dye in absence of salt and dyeing behaviour of which showed Langmuir-type adsorption curve that meant ionic bond was formed between fiber and HGCF. Finally results of color fastness and color strength would meet the market demand.Of late, Liu and Yao [27] transpired thiourea grafted cotton fiber where fabric that was treated with a mixture of epichlorohydrin and sodium hydroxide to produce epoxidized cotton fiber which subsequently immersed in a mixture of thiourea and sodium hydroxide. The chemistry involved here was epoxy groups of epoxidized cotton fiber responds to amino groups of thiourea which was obtained from FTIR spectrum and XPS. Among of all treatment condition, 10 g/l thiourea, 6 g/l NaOH, 40°C temperature and 60 min were more favorable for grafting cotton fabric. All results such as color strength, wash fastness levelness obtained from dyeing of thiourea grafted cotton fiber were satisfactory.
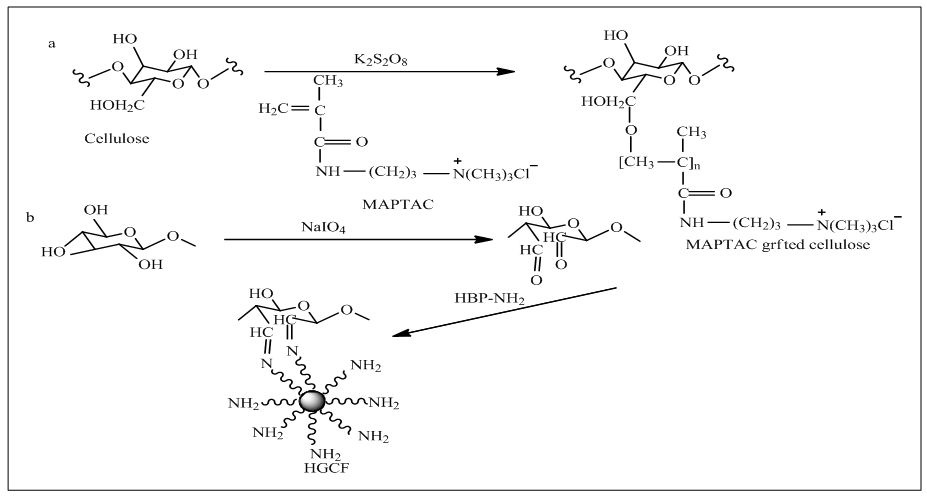 | Figure 6. a) Preparation of MAPTAC grafted cellulose [25] b) Preparation of HBP-NH2 grafted cotton fiber [26] |
2.1.5. Modification by Cationic Monomer
- Already, a lot of cationic monomers were used to cationize cotton fiber among which glytac A, CHPTAC, 1-chloro-2-hydroxy-3-trimethylammonium propane, Betaine etc. are important. Glytac AAll of them, glytac A was one of the earlier cationic agent that attract the sight first, commonly known as glycidyl trimethylammonium chloride or 2,3-epoxypropyl-trimethylammonium chloride (Fig. 7a), a popular epoxy compound mentioned in a number of papers for zero electrolyte dyeing [5, 28]. Among them, modification of cellulose with pad batch method using glytac A was important because of having versatile amenities from modified fabric such as electrolyte free dyeing, excellent color fastness and color strength reported by Hauser and Tabba [29]. Here, padding of fabric was carried out with a mixture of 50 g/l 2,3-epoxypropyl trimethyl ammonium chloride (65% solution) and 36 g/l (50%) sodium hydroxide , then batched for 24 h at room temperature.Epichlorohydrin precursor of glytac A Hindmost, epichlorohydrin precursor of glytac A structurally named as 3-chloro-2-hydroxypropyl trimethyl ammonium chloride (CHPTAC) (Fig. 7b) was reported which was converted to epoxy propyl trimethyl ammonium chloride (EPTAC) under alkaline condition [30] widely used for rendering cationic charge to the cotton, assisted to form ionic bonds with negatively charged reactive dye, thus paved the way of salt-free dyeing [31].Initially, Montazer et al., [32] usedpad-batch process to modify the cotton woven fabric pursued by dyeing with exhaust method. A mixture of 35g/l CHPTAC and 15g/l sodium hydroxide was applied for pretreatment with a pick up % was 100. Results of exhaustion, fixation and color strength were better than that of untreated one’s due to strong attraction between positive sites on modified cotton and anionic dyes. Moreover, crock fastness and rubbing fastness for both treated and untreated gave similar result whereas, light fastness was excellent, as CHPTAC penetrated into cotton fiber before fixation. Though, reaction efficiency was increased in this process, wastage of CHPTAC was still a great problem.To resolve the problem, Wang et al., [33] proposed two bath pad-bake processes for treatment of CHPTAC and alkali in two separate baths so that recycle of CHPTAC could be possible and cationization could be done continuously which reduced effluent discharge and gave salt-free dyeing. Preferred condition for padding was 8% (owf of batch) of CHPTAC and 1.8% (owf batch) of sodium hydroxide following baked at 60°C for 6 min. Also obtained results met the current market demand.Afterwards, Fu et al., [34] experimented simultaneous mercerization and cationization with CHPTAC in pad (100% pick up)-batch (room temperature for 20 hour) and proved successful in enhancing depth of shade on cotton reactive dyeing without salt addition lead to an environment benign process.Lately, Nallathambi and Rengaswami [35] focused on exhaust method to apply CHPTAC on knit cotton hosiery fabric or industrial application. Cotton bleached was treated with NaOH followed by CHPTACmolar ratio of which was 1:2 at 40°C for 10 min with a liquor ratio of 1:6. Here, alkali was required to form soda cellulose with cellulose, hydroxyl groups of which reacted with EPTAC obtained from CHPTAC, thus produced cationized cotton. Obtained exhaustion, fixation and color strength were twice than that of conventional method.1-chloro-2-hydroxy-3-trimethylammonium propaneBesides, Wang and Lewis [36] reported a non-toxic fiber reactive compound named 1-chloro-2-hydroxy-3-trimethylammonium chloride (Fig. 7c) was inaugurated from α-aminoalkyl-ω-quaternary-amine compound and acryloyl chloride. The cotton fabric was immersed in a solution of 50g/l AAHTAPC and 30g/l NaOH by padding to provide 90% wet pick up and baked in a beaker at 125°C for 6 min which were mentioned as optimum condition for pretreatment. Actual mechanism involved during modification was attachment of hydroxyl groups of cellulose with double bond of AAHTAPC. Treated cotton was dyed with 20 different reactive dyes at boiling temperature for 60 minutes in exhaust method. The results of exhaustion, fixation and color yield of treated cotton were higher than that of untreated ones in absence of salt owing to containing lots of cationic charge which form ionic bond with cellulose. But a great disadvantage of dyeing of AAHTAPC modified cotton was the tendency of ring dyeing as dye molecules quickly tied into specific quaternary sites.Betaine (N, N, N-trimethyl glycine)The aforementioned problem could be eliminated by using another quaternary ammonium compound, Betaine chemically known as N, N, N-trimethyl glycine (Fig. 7d). In presence of acid, Betaine turned into betaine hydrochloride which formed ester bond with cotton by reaction of carboxyl group of betaine hydrochloride and hydroxyl groups of cotton. Cotton fabrics were immersed into a solution containing betain, hydrochloric acid and dicyandiamide with 90% pick up and dried at 80°C for 3 min, baked at 150°C for 40s. Obtained result showed the reduction of tensile strength of cationized cotton as modification was carried out under acidic and high temperature. Besides color strength, fastness properties were also satisfactory level. Major merit came out here was the removal of cationic group from fabric under steam fixation condition [37].Other cationic monomersThere are more amino compounds such as EDTA, DMEA except quartenary ammonium compound those are also used for rendering positive charge to cellulose.A mixture of 8% EDTA(Fig.7e) in according with 8% potassium sodium tartrate was applied in pad-dry-cure technique where drying at 95°C for 5 min and curing at 140°C for 5 min to obtain optimum results of exhaustion and fixation. Actually, mechanism involved here was formation of ester linkage between carboxyl group of EDTA and hydroxyl groups of cellulose. Here, PH was maintained in a way that the dyeing commenced at PH 4 due to protonation of amino group for achieving higher exhaustion followed by increasing PH 11 for fixation more than 90%. Satisfactory dyeing properties lead to this process for commercialization [38]. Another cationic monomer chemically known as 2-choloro-2-dimethylaminoethyl hydrochloride (Fig.7f) was also induced to provide amino group in cellulose structure which also gives positive charge in all cases [39].
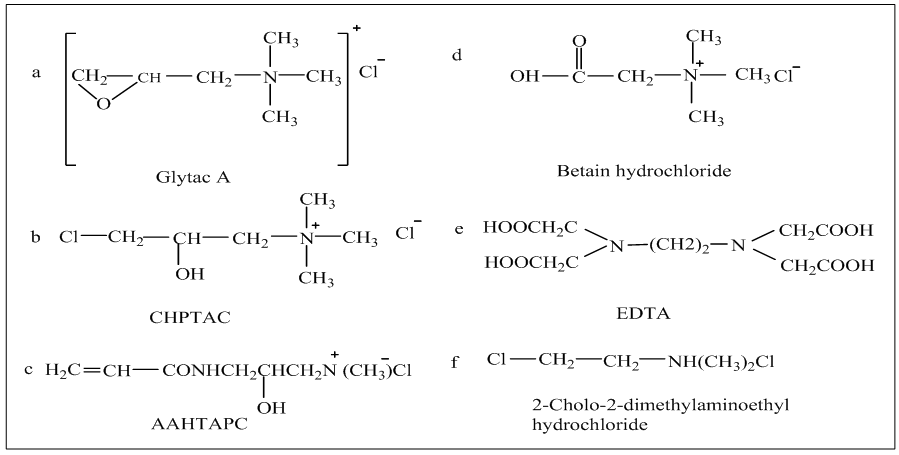 | Figure 7. Different cationic monomer a[5]b[31]c[36]d[37]e[38]f[39] |
2.1.6. Modification by Cationic Polymer
- Though synthetic monomers have the ability to penetrate easily into fiber to ensure improved exhaustion and fixation, they show less substantivity to the fiber for which a large amount of them is required to apply which limit their application [11]. To minimize such problems synthetic polymers are now experimented, available few of them are discussed here.Polyepichlorohydrin resin Burkinshaw et al., [40] used Polyepichlorohydrin resin commercially known as Hercosett 125 was to modify cotton so that salt and alkali free reactive dyeing could be achieved. Primarily, condensation of adipic acid with diethylenetriamine continued by reaction and some cross-linking with epichlorohydrin gave poly epichlorohydrin resin which contained azetidinium groups, reacted with nucleophilic sites in cotton to offer opportunity of reactivity and substantivity for cotton. This pretreatment could be done by pad dry process followed by dyeing in exhaust method. Dye uptake was better than untreated one. Moreover, dye fixation reached maximum at PH 7 which ensured alkali free dyeing. Besides quality investigation ensured excellent wash fastness though light fastness was not so good. One of major merits mentioned here was absence of ring dyeing due to migration of dye to the yarn surface due to low substantivity of it’s for cotton.Poly (4-vinyl pyridine) quaternary ammonium compoundLatter, Blackburn and Burkinshaw [41] produced Poly (4-vinyl pyridine) quaternary ammonium compound that was emerged from 4- vinyl pyridine and 1-amino-2- chloroethane, was isolated from above mentioned polymer in case of attachment process of it’s with cotton. Here, modification agent adsorbed into the fiber not only by H- bonding formed between hydrogen atom of hydroxyl groups of cellulose and
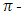 electron of unquaternized pyridine rings in modifying agent, but also ion-dipole interaction formed between positively charged nitrogen atoms of pyridinum rings and lone pair of electrons on the oxygen atom of hydroxyl groups of cellulose. The cationic sites of polymer enabled the anionic dye to attract in absence of salt. Moreover, dyes were fixed with amino functional nucleophiles at neutral pH so that dye hydrolysis could be reduced. Besides, both pretreatments and dyeing were carried out in exhaust method owing to higher molecular weight and substantivity of polymer. Obtained wash fastness displayed excellent after one wash that enables to save water, time and also for reducing hydrolysis cost also saved. Ultimately, it offered an eco-friendly dyeing process.Cationic diblock copolymerHan et al., [42] introduced cationic diblock copolymer (Fig. 8a), a quaternary ammonium compound executed from [2-methacryloyloxy) ethyl] trimethylammonium chloride to produce cationized fiber so that it could replace the use of salt. Pretreatment with PEO45-MeDMA diblock copolymer was carried out at 60°C for 1 hour in exhaust method continued dyeing at 60°C for 45 min at 1:10 liquor ratio. Findings showed that up to 5 g/l concentration of copolymer, dye uptake increased due to formation of ionic bond between dye and fiber. When concentration exceeds 10g/l dye uptake reduced as they reduced free dye. Moreover, sorption isotherm showed Langmuir-type sorption mechanism which gave strong evidence of ionic bond.Poly (Vinylamine chloride) Ma et al., [43] inaugurated another polymer contained primary amino group had been reported as surface modifier of cotton because of presence of large number of cationic sites (NH3+ Cl̄) simplified for encouraging salt-free dyeing. Particularly, pad-air dry-bake method was effective to apply PVAmHCl (Fig. 8b) evenly and bind firmly to cotton where padding involved 5 g/l PVAmHCl applied by 2 dips and 2 nips process with a wet pick up of 80% at pH 7 subsequently baked at 100°C for 10 min in a baker. Further, effect of concentration concluded that exhaustion increase up to 10g/l, after exceeding it exhaustion decreased as a result of weak bonding between cellulose and cationic polymer due to higher amount of PVAmHCl created repulsion force within polymers. Exhaustion, fixation, color fastness to wash and rubbing of exhaust dyed pretreated cotton showed excellent properties which increased its commercial importance. Besides, gained Langmuir type adsorption isotherm confirmed the ionic attraction between cationic (NH3+ Cl̄) of PVAmHCl and anionic reactive dye.Tertiary amine cationic polyacrylamideThough above three compound showed a great deal of advantages, they also gave different disadvantages such as the presence of primary and secondary amine could change the hue of dyes, polymeric quaternary ammonium salts affected the wet fastness properties of dyed cotton strong due to electrostatic attraction between fiber and hydrolyzed dye. The aforementioned obstacles could be eliminated by using Tertiary amine cationic polyacrylamide (Fig. 8c) employed by Xiaoxu et al., [44]. Dip-pad-bake method was employed to modify cotton so that it could form electrostatic interaction with cotton during exhaust dyeing at 20°C. Optimum condition found here for preparation was immerging the sample at 20°C for 2 min padding mangle with pick up% of 80%, subsequently baked at 100°C for 5 min. It was found fixation, exhaustion and reactivity of dyes depended on appropriate molecular mass (M W = 1.1X 104 g.mol-1) and concentration of TACPAM. Higher molecular mass resulted in higher exhaustion, but lower fixation as higher molecular mass of modifying agent affected the action between treated cotton and dye and penetrate ability of dye. Achieved result suggested that the dye ability like color strength, fixation and fastness properties are marketed standard. Polyamino carboxylic acidRecently a compound named Polyamino carboxylic acid (Fig. 8d) was used by Ameri Dehabadi et al., [45] for above mentioned purpose which contained two functional group named carboxyl group and amine groups. Carboxyl group involved to form ester linkage with cellulose and protonation of amino group helped for creating cationized cotton. Fabric was pretreated using exhaustion and pad-dry-cure technique subsequently dyed with exhaust method. Dyeing performance like color strength of treated cotton fabric was 3 times higher than that of untreated cotton and also washing and rubbing fastness were not affected.
electron of unquaternized pyridine rings in modifying agent, but also ion-dipole interaction formed between positively charged nitrogen atoms of pyridinum rings and lone pair of electrons on the oxygen atom of hydroxyl groups of cellulose. The cationic sites of polymer enabled the anionic dye to attract in absence of salt. Moreover, dyes were fixed with amino functional nucleophiles at neutral pH so that dye hydrolysis could be reduced. Besides, both pretreatments and dyeing were carried out in exhaust method owing to higher molecular weight and substantivity of polymer. Obtained wash fastness displayed excellent after one wash that enables to save water, time and also for reducing hydrolysis cost also saved. Ultimately, it offered an eco-friendly dyeing process.Cationic diblock copolymerHan et al., [42] introduced cationic diblock copolymer (Fig. 8a), a quaternary ammonium compound executed from [2-methacryloyloxy) ethyl] trimethylammonium chloride to produce cationized fiber so that it could replace the use of salt. Pretreatment with PEO45-MeDMA diblock copolymer was carried out at 60°C for 1 hour in exhaust method continued dyeing at 60°C for 45 min at 1:10 liquor ratio. Findings showed that up to 5 g/l concentration of copolymer, dye uptake increased due to formation of ionic bond between dye and fiber. When concentration exceeds 10g/l dye uptake reduced as they reduced free dye. Moreover, sorption isotherm showed Langmuir-type sorption mechanism which gave strong evidence of ionic bond.Poly (Vinylamine chloride) Ma et al., [43] inaugurated another polymer contained primary amino group had been reported as surface modifier of cotton because of presence of large number of cationic sites (NH3+ Cl̄) simplified for encouraging salt-free dyeing. Particularly, pad-air dry-bake method was effective to apply PVAmHCl (Fig. 8b) evenly and bind firmly to cotton where padding involved 5 g/l PVAmHCl applied by 2 dips and 2 nips process with a wet pick up of 80% at pH 7 subsequently baked at 100°C for 10 min in a baker. Further, effect of concentration concluded that exhaustion increase up to 10g/l, after exceeding it exhaustion decreased as a result of weak bonding between cellulose and cationic polymer due to higher amount of PVAmHCl created repulsion force within polymers. Exhaustion, fixation, color fastness to wash and rubbing of exhaust dyed pretreated cotton showed excellent properties which increased its commercial importance. Besides, gained Langmuir type adsorption isotherm confirmed the ionic attraction between cationic (NH3+ Cl̄) of PVAmHCl and anionic reactive dye.Tertiary amine cationic polyacrylamideThough above three compound showed a great deal of advantages, they also gave different disadvantages such as the presence of primary and secondary amine could change the hue of dyes, polymeric quaternary ammonium salts affected the wet fastness properties of dyed cotton strong due to electrostatic attraction between fiber and hydrolyzed dye. The aforementioned obstacles could be eliminated by using Tertiary amine cationic polyacrylamide (Fig. 8c) employed by Xiaoxu et al., [44]. Dip-pad-bake method was employed to modify cotton so that it could form electrostatic interaction with cotton during exhaust dyeing at 20°C. Optimum condition found here for preparation was immerging the sample at 20°C for 2 min padding mangle with pick up% of 80%, subsequently baked at 100°C for 5 min. It was found fixation, exhaustion and reactivity of dyes depended on appropriate molecular mass (M W = 1.1X 104 g.mol-1) and concentration of TACPAM. Higher molecular mass resulted in higher exhaustion, but lower fixation as higher molecular mass of modifying agent affected the action between treated cotton and dye and penetrate ability of dye. Achieved result suggested that the dye ability like color strength, fixation and fastness properties are marketed standard. Polyamino carboxylic acidRecently a compound named Polyamino carboxylic acid (Fig. 8d) was used by Ameri Dehabadi et al., [45] for above mentioned purpose which contained two functional group named carboxyl group and amine groups. Carboxyl group involved to form ester linkage with cellulose and protonation of amino group helped for creating cationized cotton. Fabric was pretreated using exhaustion and pad-dry-cure technique subsequently dyed with exhaust method. Dyeing performance like color strength of treated cotton fabric was 3 times higher than that of untreated cotton and also washing and rubbing fastness were not affected.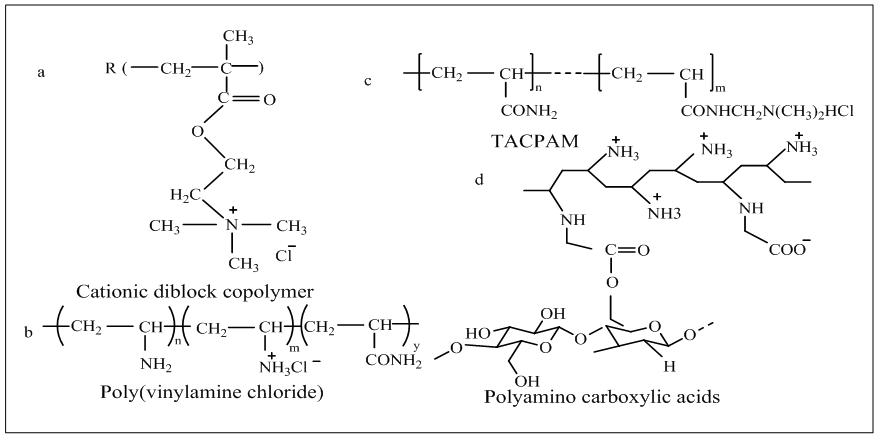 | Figure 8. Various types of cationic polymer a[42]b[43]c[44]d[45] |
2.2. Progress of Cationic Reactive Dye
- A small number of zero-salt fiber reactive dyes are shaped recently but still they are not commercially released. It is predicted that they will catch the market soon due to high environment pollution concern throughout the world as well as offer more advantages over conventional dyes. The progress of such novel dyes are considered underneath:
2.2.1. Zero-Salt Fiber Reactive Vinyl Sulphone Dye
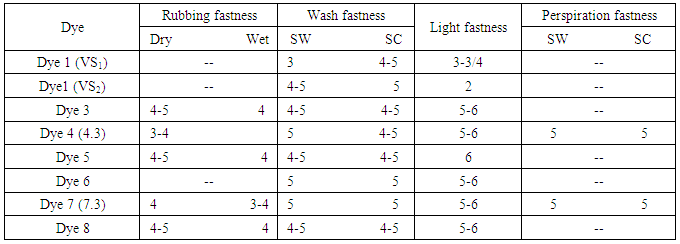
- Hinks et al., [46] initiated some reactive dyes those contained vinyl sulphone group, was incorporated with cationic pyridinium and bis-pyridinium chloride for creating cationic fiber reactive dye (dye 1). Here, dyes are indicated by VS1 and VS2. The chemical structures of such dyes are illustrated in figure 9.During dyeing, elimination of salt was achieved due to presence of cationic charge in the dye structure. Moreover, covalent bond, van der walls forces, hydrogen bonding were created between dye and fiber. Thus both facilitatedinnate substantivity of cationic dyes towards negatively charged cotton.
 | Figure 9. Aforementioned structure of cationic reactive dye (Dye1[46]Dye2[47]Dye3[48]Dye4[49]Dye5[50, 48]Dye6[51]Dye7[52]Dye8 [48]) |
2.2.2. Triazinyl Quaternary Ammonium Cationic Reactive Dye
- Zhao et al., [47] established dye 2, a zero-salt cationic reactive dye that contained anthraquinone group as chromophore that provide stability of dye structure and quaternary ammonium structure to impart solubility and increase interaction to cotton in absence of salt. Here another advantages obtained was that QAS contained long chain alkyl groups (12 carbons) that increase antimicrobial power. However, dyeing performance was good except wash fastness of antimicrobial functioned dyed fabric.Dye 3 is also a cationic reactive dye which contains quaternary ammonium group as soluble bits and mono chlorotriazine as reactive group same as previous one but it does not contain long chain alkyl group in its structure. The presence of quaternary ammonium group provided substantivity to anionic cotton fiber that leads to salt-free dyeing [48]. Again, sufficient water solubility was not achieved from one quarternary amino group alone. To solve this problem, polyether amine was embodied into triazynyl group of reactive dye with one quaternaty ammonium chlorides. Thus a series of new cationic reactive dye (dye 4) was initiated in which quaternaty ammonium and polyether amine performed as soluble moietry and MCT act as a reactive group. These dyes provided positive result in case of all properties like substantivity, solubility, exhaustion, fixation fastness and leveling properties [49]. Besides, synthesis of series of monofluorotriazine quaternary ammonium cationic reactive dye has been introduced [50]. Among them dye 5 was used for salt-free dyeing of cotton and released a huge amount of fluoride ion after reaction that had negative impacts on marine life [48].
2.2.3. Triazinyl Quaternary Ammonium Pyridinium Chloride Cationic Reactive Dye
- To offer zero electrolytes dyeing, Srikulkit and Santifuengkul [51] introduced a model cationic reactive dye (dye 6) which contained N-(2-amino ethyl) pyridinium chloride into chlorotriazine group. Pyridinium chloride acts as cationic group which attract the negatively charged cellulose and thus assists to perform salt-free dyeing. Moreover, it facilitated by being removed from fabric after dyeing to improve light fastness properties of dyed fabric with significant change of hue.Latter, by incorporation of two quaternary ammonium salt groups into triazinyl group of reactive dye (Dye 7), series of novel cationic dyes had been discovered which also contained an epoxy group act as reactive group and thus combined nucleophiles on the cotton covalently. Excellent exhaustion of dye on cotton was achieved as a result of existence of two cationic groups in dye structure. Though such types of reactive dyes had higher solubility due to two cationic groups, they gave poor leveling properties [52].
2.2.4. Nicotinic Acid Quaternary Triazine Cationic Reactive Dye
- In 1979, quaternised nicotinic acid derivatives were introduced in reactive group. Latter, Nippon Kayacku introduced Kayacelon reacts and observed that for fixation of bis-nicotinic quaternary ammonium reactive dyes, alkali was not essential and termed as neutral fixing Reactive dye [9]. Then attempt has been taken for synthesis of salt-free, alkali free dyes to protect the environment.Recently, Zheng et al., [52] synthesized dye 8, was a novel nicotinic acid quaternary triazine cationic reactive dye used for salt and alkali free dyeing of cotton fabric, having quaternary ammonium group as soluble moieties and nicotinic acid quaternary triazine as reactive group to form covalent bond with cotton. Nicotinic acid was formed as a by-product after dyeing which have no harmful effect on environment.
|
|
3. Conclusions
- Attainable cationization process including both bring to bear of cationic agent such as chitosan and it’s derivatives, starch, dendrimer, cationic monomer and polymer and cationic reactive dyes are narrated in this review paper with their merits and demerits to eject salt usage which lead to reduce the effluent pollution.Among cationic agent, biodegradable polymer like chitosan and starch may be most attractive alternative of salt usage especially fiber reactive chitosan, chitosan dendrimer hybrid and cationic hydrolyzed starch due to their ability to meet current market demand with sustainable environmental approach. Besides, grafting of cellulose with thiourea and HPB-NH2 may be another adoptable process for cationization as fastness properties and color strength are satisfactory.Recently, to bring down the repulsion effect between anionic reactive dye and cellulose, development of cationic reactive dyes is being raveled. A number of cationic fibers reactive zero salt dyes are designed which contains quaternary ammonium group, pyridium chloride as cationic part of dyes. Among mentioned of all dyes, nicotinic acid quaternary triazine cationic reactive dyes may be preferred one because of having versatile advantages.However, both aforementioned processes for zero salt dyes are not still commercialized. Indeed, cationization with cationic agent requires more extra pretreatment step which may lead extra cost. So use of cationic reactive dye may have possibilities to catch future market due to growing concern for pollution free dyeing.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML