-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Textile Science
p-ISSN: 2325-0119 e-ISSN: 2325-0100
2017; 6(1): 15-19
doi:10.5923/j.textile.20170601.03

Mercerization of Cotton Yarn Fibers. Optimization of Caustic Soda Concentration via Degree of Mercerization, Dyability and Mechanical Properties
1Department of Chemistry, Faculty of Science, Taif University, Taif, Saudi Arabia
2Department of Chemical Engineering, Higher Institute for Engineering and Technology, New Damietta, Egypt
Correspondence to: Mohamed E. Khalifa, Department of Chemistry, Faculty of Science, Taif University, Taif, Saudi Arabia.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Merecerization treatment for cotton yarn fibers is very essential process to increase the luster, tensile strength and enhancement their dyability. In this investigation, a selection of three common yarn patterns (2/9.5, 984 and 3/8) was subjected to different mercerization experiments under factory conditions, to optimize the caustic soda concentration for highest quality and economic impact. Each of mechanical and color properties were measured, where the experimental evidences indicated that optimum benefits of caustic soda concentration for improving color yield and mechanical properties can be obtained at 25% caustic soda concentration. The retrieval study of the wasted caustic soda was also performed.
Keywords: Cotton yarns, Mercerization, Luster, Caustic soda, Dyability
Cite this paper: Mohamed E. Khalifa, Mercerization of Cotton Yarn Fibers. Optimization of Caustic Soda Concentration via Degree of Mercerization, Dyability and Mechanical Properties, International Journal of Textile Science, Vol. 6 No. 1, 2017, pp. 15-19. doi: 10.5923/j.textile.20170601.03.
Article Outline
1. Introduction
- Mercerization treatment process is subjecting the vegetable fibres (e.g. Cotton) to the action of a concentrated solution of a strong base at a proper temperature, to produce great swelling with resultant changes in the fiber structure. In industry, mercerization of cotton fabrics and yarn fibers is an essential preparation process in wet-wet or wet-dry processing technology for treating textile materials that enhances dyeing behaviour besides improving dimensional stability, tensile strength, and luster as well [1-4]. Changes in microstructure, morphology, and conformation of the cellulose chains also occur during mercerization depending on the treatment processes, mechanisms of their actions, temperature, duration, as well as the degree of applied tension during the treatment and physical state of cellulose. Cotton yarn mercerization can be applied in hank, cheese, single-end mercerization, or tow mercerization on raw or scoured, wet or dry. Hank mercerization process is carried out by slack or tension treatment, but combination of slack and tension treatment is usually done to prevent a loss in yardage and to produce the high luster [5-10]. Upon treating the cotton fibers with caustic soda (sodium hydroxide), they swell laterally and shrink longitudinally due to diffusion of water and alkali, and by application of tension to the yarn in the swollen state, a permanent luster is developed, while in the absence of tension, the resulting product gains in elasticity and becomes suitable for use as a stretch yarn or fabric [11]. The degree of swelling depends on the concentration of caustic soda, where at low caustic soda concentration, the sodium hydroxide molecules are hydrated by large number of water molecules and the diameter of the hydrated ions is too large to penetrate into the macromolecular structure of cotton, while increasing of its concentration will decrease the number of water molecules available for the formation of hydrates and thus formation of hydrated ion pairs which are capable of penetrating into the fiber structure of cotton by breaking hydrogen bonds and weak Van der Waal forces between cellulose chains resulting in fiber swelling and the corresponding fiber shrinkage [12-14]. The main objective of this study is to establish experimentally, under factory conditions and in production scale, the most appropriate concentration of caustic soda to be applied for selected patterns of industrially important cotton yarns at which higher degree of mercerization, mechanical properties and dyability are achieved as a research study in Misr Beida Dyers factory, Egypt.
2. Results and Discussion
- The fibre cell structure reflects the incident light as per its cell structure in a regular or irregular way, thus affecting the luster of the fibre surface. The regular reflection from the fibre is obtained by passing through a mercerizing treatment, where the cotton fibres' shape become round when it shrinks in the solution of caustic soda (figure 1). As the result of reducing the cell lumen and increasing the cell wall, both the luster and the swelling power of the fibre increase, and the strength of the fibre increases up to 20-30% as well.
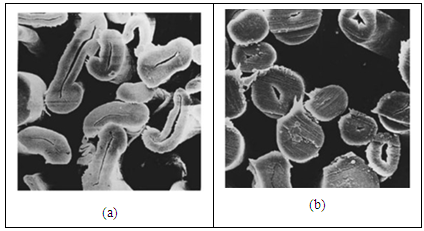 | Figure 1. Electron microscope image of cotton fibers (x2200), a, natural; b, mercerized cotton |
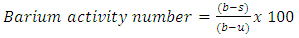 | (1) |
|
|
|
|
3. Experimental
3.1. Material and Methods
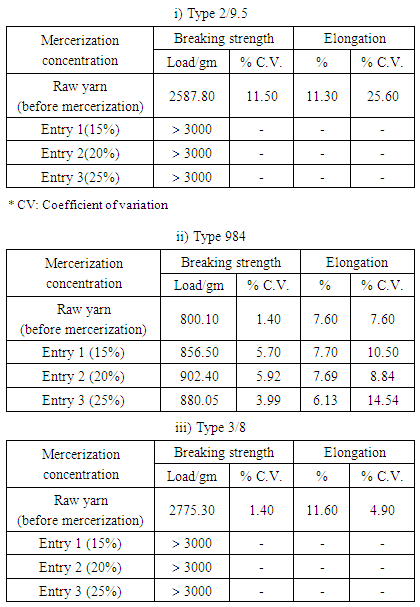
- All chemicals and auxiliaries are of commercial grade, and purchased from local (Egyptian) and international companies. Yarn fibers with three different spinning types 2/9.5, 984 and 3/8 were supplied by Kafr El-Dawar for spinning and weaving Co., Bohaira, Egypt. Mercerization experiments were performed in production scale using Jaeglli Hank Mercerizer (Jaeggli-Meccanotessile Co., Italy). Tensile strength and elongation were measured on Tenso lab tester (Mesdan Co., Italy) in an environment at 65% humidity and 21°C. The dye-uptake measurements for the dyed cotton yarns were carried out using a reflectance spectrophotometer (GretagMacbeth CE 7000a, GretagMacbeth Co., Windsor, UK). Fastness to washing was carried out using the automatic Rota dyer launder (Texcare Co., Delhi, India), fastness to perspiration was assessed according to the test sponsored by the British Standard Institute-Society of Dyers and Colourists (BSS), and fastness to light was carried out using the “Weather-o-meter” (Atlas Electric Devices Co., Chicago, IL, USA). All production experiments, chemical, physical tests, dyeing process and colorimetric measurements were performed by the yarn factory and chemical laboratories of Misr Beida Dyers Co., Alexandria, Egypt and in accordance to the American Association of Textile Chemists and Colorists (AATCC) standard methods.
3.2. Method of Application
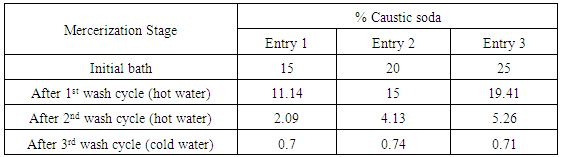
- The three types of yarn fibers were mercerized at 15%, 20%, 25% and 30% of caustic soda solutions in four separate entries in presence of an anionic wetting agent (Floranit 4028, product of Henkel Co., Germany), at 18°C for 120 sec. immersion time under the tension of the machine. The hanks were extended by application of tension to the original length, rinsed with hot water (80°C) for two times and cold water (18°C) for 60 sec., neutralized using 10% acetic acid solution and finally rinsed with cold water. The mercerized cotton yarns in form of hanks were centrifuged and dried by hot air drying room. The yarns were bleached by hydrogen peroxide bleaching bath at 100C for 1 hour, and finally dyed using reactive dye (Levafix Royal Blue E-FR, supplied by Dystar Co., Singapore) at 40C for 90 min. by reactive dyeing technique.
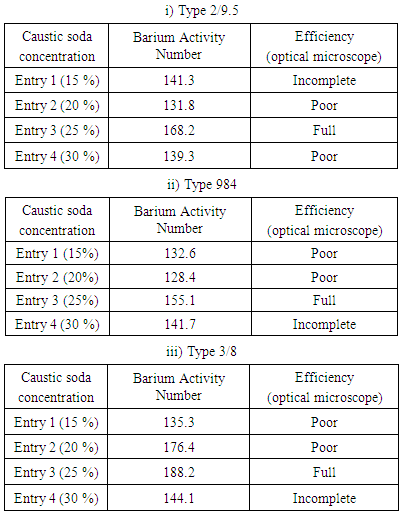
3.3. Barium Activity Number Method [18]
- One gram of mercerized and unmercerized cotton yarns were each cut into small pieces, dried over phosphorous pentoxide for 5 h, then conditioned at 65 % relative humidity at 31C before testing and consequently treated with 30 ml of 0.25 N barium hydroxide solution in 100 ml conical flasks. After 2 h, 10 ml of the solution is titrated against 0.1 N hydrochloric acid. A blank was also run without any yarn sample. The titration readings of the blank (b), mercerized (s) and unmercerized (u) samples, were recorded and the barium activity number values are calculated using equation 1. Appropriately large numbers of conditioned samples were taken, considering the moisture regain of the samples. Thus, if the moisture regains 8%, 1.08 g of the conditioned cotton sample was weighted to get 1g of the bone-dry cotton.
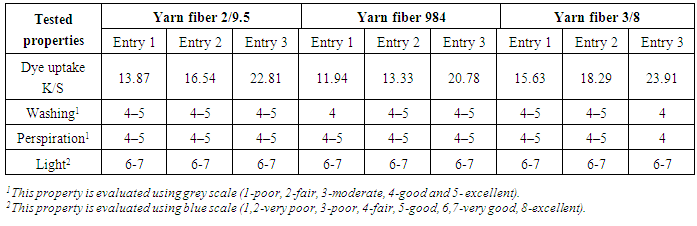
3.4. Dyeing Method and Color Fastness
- For dyeing cotton fibers, in practical terms, reactive dyes are suitable. Levafix Royal Blue E-FR dyestuff was applied to the selected cotton yarns by 2% shade (o.w.f.) in presence of 50 gm/l glauber salt (Na2SO4.10H2O) and 15 gm/l soda ash (Na2CO3). The cotton yarns were exhausted in the dye bath at 40°C for 90 min. The dyes fibers were then soaped using non-ionic detergent, washed by hot and cold water intervalley and dried by hot air. The color fastness properties of the dyed fibres to washing, perspiration and light were evaluated using standard methods. The staining of adjacent cotton fabrics was assessed using the grey scale: 1-poor, 2-fair, 3-moderate, 4-good and 5-excellent, other than light fastness which scaled from 1–8 on the grey scale.
3.5. Color Yield Assessment
- The color yield of the cotton yarn fibers (Dye-uptake) was determined in term of K/S values given by the reflectance spectrometer at λmax according to the Kubelka–Munk equation: K/S= (1 − R)2/2R, where K= absorbance coefficient, S= scattering coefficient, R= reflectance ratio, where the measurement of K/S expressed the comparative color yield or dye uptake and not the absolute quantity of dye present on dyed sample.
4. Conclusions
- The mercerization process of different common types of cotton yarns showed that the degree of mercerization, dyability and tensile strength are gradually increased by increasing caustic soda concentration, while elongation character is not extremely changed. The optimum caustic soda concentration for efficient mercerization is 25% which produces the best mercerizing degree and mechanical properties rather than highest dyability based upon dye-uptake values.
ACKNOWLEDGEMENTS
- The author wishes to extend his sincere thanks and appreciation to the management and staff members of the Chemical laboratory and Yarn sector, Misr Bieda Dyes Co., Alexandria, Egypt for their technical support and great cooperation.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML