Md. Eman Talukder1, Md. Kamruzzaman1, Mithon Majumder2, Md. Shakhawat Hossain Rony2, Monoroma Hossain2, Santanu Das1
1College of Textile Chemistry & Chemical Engineering, Wuhan Textile University, Wuhan, China
2College of Textile Science & Engineering, Wuhan Textile University, Wuhan, China
Correspondence to: Md. Eman Talukder, College of Textile Chemistry & Chemical Engineering, Wuhan Textile University, Wuhan, China.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Reactive dyes square is measured very fashionable for clothes as they're environmentally safe and having sensible overall fastness properties. However application of those dyes needs an awfully high concentration of salt. The salt discharged from garment coloring is increased salinity in drain water stream that incorporates a negative impact on environmental ecology. The current work aims to eliminate the usage of salt throughout coloring of cotton merchandise with reactive dyes. The method of the concentration of salt in cotton coloring was supported the principle of cationisation (to develop a positive charge) of cotton. The treated cotton once bleached from slightly acidic tub generates positive sites attributable to protonation within the amino. The reactive dyes being anionic (negatively charged) in resolution get interested in the positive charges on the fiber that eliminates the salt needs for satisfactory dye exhaustion. After finished our work we found that Salt concentration for reactive dyes on textile materials is more important to show the good colorfastness, wash fastness, rubbing fastness.
Keywords:
Cotton, Salt, Reactive Dyes, Fabrics, Effect
Cite this paper: Md. Eman Talukder, Md. Kamruzzaman, Mithon Majumder, Md. Shakhawat Hossain Rony, Monoroma Hossain, Santanu Das, Effects of Salt Concentration on the Dyeing of Various Cotton Fabrics with Reactive Dyes, International Journal of Textile Science, Vol. 6 No. 1, 2017, pp. 7-14. doi: 10.5923/j.textile.20170601.02.
1. Introduction
The physical-chemical necessity of the utilization of salt and soda in reactive coloring is standard to any or all technicians. The salt is Associate in nursing exhausting agent to push the color towards polysaccharide molecules and therefore the alkali (soda ash) is hydrolyzing/fixing agent for the reactive color.In current follow, plastic fibers are preponderantly colored with reactive dyes within the presence of a substantial quantity of salt and glued underneath alkalescent conditions. However, dye fixation potency on plastic fibres is usually low (varying from 50%-90%). This, ends up in an extremely colored dye effluent that is unfavorable on environmental grounds. What is more, the high concentrations (40-100g/L) of solution and alkali (5-20 g/L) needed in polysaccharide fiber coloring might create further effluent issues [1]. During this work, a brand new fiber modification technique supported ion acrylic polymer. Pretreatment of synthetic fiber with chemical compound is believed to supply a chance for increasing each the substantively and reactivity of fibers towards reactive dyes underneath neutral conditions [1]. The character of a reactive chemical compound organic compound is specified it's going to react with nucleophile sites in synthetic fibers or within the chemical compound itself, therefore fixing the chemical compound to the substrate. Throughout future coloring, any reactions between the chemical compound and therefore the color, the fiber and therefore the color, and therefore the fiber and therefore the chemical compound and might be expected to require place, forming crosslink inside the fibers [2]. Salt may be a mineral comprised chiefly of the two components, metallic element and chloride. Common salt or common salt is that the substance NaCl, composed of the weather metallic element and chloride. Common salt crystals are solid in kind. Salt consists of small cubes tightly sure along through ionic bonding of the metallic element and chloride ions. The salt crystal is commonly used as Associate in nursing example of crystalline structure [3] so as to know the depth of the topic, one ought to perceive the fundamentals behind the term "salt" with relation to textile process. The textile substrate and dye molecule, not essentially ought to have of uniform characteristics to mix with one another. In such case, we tend to need some catalyst to facilitate colouring action on material. Salt plays this significant role of catalyst. Salt has an especially high affinity for water. Generally, Salt is critical in three ways, firstly, to drive dye into textile throughout the colouring method in textile. Secondly, use of salt results in most exhaustion of dye molecules throughout coloring method in textiles [4]. Third it's used as Associate in nursing solution for migration, sorption and fixation of the coloring material to the polysaccharide material. Salt increase the exhaustion and the attraction between the dye molecule and textile material [5]. Typically dye (direct & reactive) molecules have charges and therefore the material has also negative charge on its surface. Salt decreases the repulsion of negative-negative charges and thence improves exhaustion [6].Within the simplest terms, all reactive dyes are created from 3 basic units, a chormophore, a bridge Associate in nursing a reactive group/ teams (either a haloheteocycle or an activated double bond) [7]. These dyes are used for coloring of plastic fibers Associate in nursingd once these are applied to a plastic fiber in an alkalic dye bathtub, they kind a bond with hydroxyl of the fiber by with chemicals reacting with fiber [8]. The bond shaped between the dye molecule and fiber build dye molecule a locality of the fiber molecule [9].
2. Materials and Methods
100% grey cotton Plain weave, Single jersey & Rib (Double jersey) fabric was used in this project work. Dye, Wetting Agent, Detergent, Anticreasing Agent, Sequestering Agent, Antifoaming Agent, Salt, Alkali, Acetic Acid & Peroxide Killer. Pipette, Conical flask, GSM cutter, Balance & Dye bath Preparation of the fabric for dyeingØ The scoured and bleached fabric is taken for dyeing process with reactive dyes.Ø Then the fabric is conditioned in the room temperature.Dyeing process for 100% cotton fabric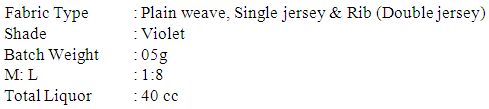 Recipe for salt requirement (15% less)
Recipe for salt requirement (15% less)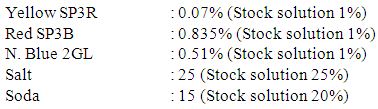 Recipe for actual salt requirement
Recipe for actual salt requirement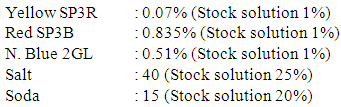 Recipe for salt requirement (15% addition)
Recipe for salt requirement (15% addition)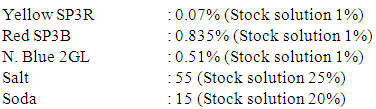 General dyes calculation formula
General dyes calculation formula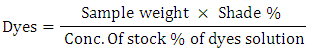
 Equation 1General chemicals calculation formula
Equation 1General chemicals calculation formula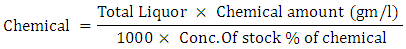
 Equation 2
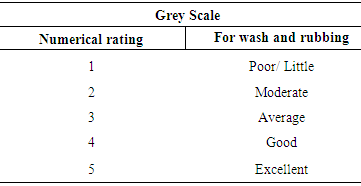
Equation 2 Method of testing Color fastness to RubbingPrincipleThis test is designed to determine the degree of color which may be transferred from the surface of a colored fabric to a specific test cloth for rubbing (dry + wet).EquipmentØ Crock meter, Cotton rubbing cotton, Grey scale, Stop watch & Color matching cabinetSize of fabric4×5 cm two pieces of sample (one warp direction/wale direction & another weft/course direction).Test procedureØ Lock the test specimen onto the base of the crock meter.Ø Using the spinal spring clip, set 5cm5cm of the white cotton fabric to the finger of the crock meter.Ø Lower the covered finger on the test sample.Ø Turn hand crank at the rate of one turn per second (1010 sec).Ø Remove the white rubbing test cloth and evaluate with grey scale.EvaluationEvaluation the contrast between the treated and untreated white rubbing cloth with grey scale and rated 1-5.Method of testing Color fastness to washColor fastness to wash is very important for lab-dip. There are varieties of testing procedure, because-Ø The methods depend on the use of dyed goods.The degree of fading and staining of dyed goods for washing depends upon the following factors:Ø Temperature range may be from 40°C-95°C.Ø The type and amount of detergent added to the washing bath. In many testing procedure a standard detergent is used. The washing liquor to goods ratio is 50:01.Principle of wash fastnessA specimen (lab-dip) in contact with specified adjacent fabric or fabric or fabric is laundered, rinsed and dried. The specimen/composite sample is treated under appropriate condition in a chemical bath for short time. The abrasive action is accomplished by the use of a liquor ratio and an appropriate number of steel balls. The change in color of the specimen (dyed sample) and the staining of the adjacent fabric is assessed by recommended Grey scale (1-5) [10].Apparatus and materialsØ Wash –wheel with a thermostatically controlled water bath and rating speed of (40±2) rpm.Ø Stainless steel container (capacity 55±50 ml)Ø Stainless steel ball (dia = 0.6cm, weight = 1 gm)Ø SDC, Multifibre fabric (Acetate, cotton, nylon, polyester, acrylic, wool)Ø Thermometer, Sewing machine, Dryer, Color matching cabinet and ISO Grey Scales.ReagentsØ Sodium perborate (NaBo2.H2O.3H2O)Ø ECE without brightening agent & Distilled water Test specimenTest specimen cut a sample of dyed goods 10*4cm and sew it with same size multifibre fabric. This is the composite test sample. Evaluation of wash fastnessCompare the contrast between the treated and untreated sample with Grey Scale for changing color of dyed sample and staining of adjacent fabric in a color matching cabinet. Numerical rating for color changing is the shade and staining to adjacent fabric. Number of method used.Assessment of color fastness
Method of testing Color fastness to RubbingPrincipleThis test is designed to determine the degree of color which may be transferred from the surface of a colored fabric to a specific test cloth for rubbing (dry + wet).EquipmentØ Crock meter, Cotton rubbing cotton, Grey scale, Stop watch & Color matching cabinetSize of fabric4×5 cm two pieces of sample (one warp direction/wale direction & another weft/course direction).Test procedureØ Lock the test specimen onto the base of the crock meter.Ø Using the spinal spring clip, set 5cm5cm of the white cotton fabric to the finger of the crock meter.Ø Lower the covered finger on the test sample.Ø Turn hand crank at the rate of one turn per second (1010 sec).Ø Remove the white rubbing test cloth and evaluate with grey scale.EvaluationEvaluation the contrast between the treated and untreated white rubbing cloth with grey scale and rated 1-5.Method of testing Color fastness to washColor fastness to wash is very important for lab-dip. There are varieties of testing procedure, because-Ø The methods depend on the use of dyed goods.The degree of fading and staining of dyed goods for washing depends upon the following factors:Ø Temperature range may be from 40°C-95°C.Ø The type and amount of detergent added to the washing bath. In many testing procedure a standard detergent is used. The washing liquor to goods ratio is 50:01.Principle of wash fastnessA specimen (lab-dip) in contact with specified adjacent fabric or fabric or fabric is laundered, rinsed and dried. The specimen/composite sample is treated under appropriate condition in a chemical bath for short time. The abrasive action is accomplished by the use of a liquor ratio and an appropriate number of steel balls. The change in color of the specimen (dyed sample) and the staining of the adjacent fabric is assessed by recommended Grey scale (1-5) [10].Apparatus and materialsØ Wash –wheel with a thermostatically controlled water bath and rating speed of (40±2) rpm.Ø Stainless steel container (capacity 55±50 ml)Ø Stainless steel ball (dia = 0.6cm, weight = 1 gm)Ø SDC, Multifibre fabric (Acetate, cotton, nylon, polyester, acrylic, wool)Ø Thermometer, Sewing machine, Dryer, Color matching cabinet and ISO Grey Scales.ReagentsØ Sodium perborate (NaBo2.H2O.3H2O)Ø ECE without brightening agent & Distilled water Test specimenTest specimen cut a sample of dyed goods 10*4cm and sew it with same size multifibre fabric. This is the composite test sample. Evaluation of wash fastnessCompare the contrast between the treated and untreated sample with Grey Scale for changing color of dyed sample and staining of adjacent fabric in a color matching cabinet. Numerical rating for color changing is the shade and staining to adjacent fabric. Number of method used.Assessment of color fastness Table 1. Grey Scale
 |
| |
|
3. Results and Discussion
Physical results of Plain weave and Single jersey, Rib (Double jersey) Knit fabric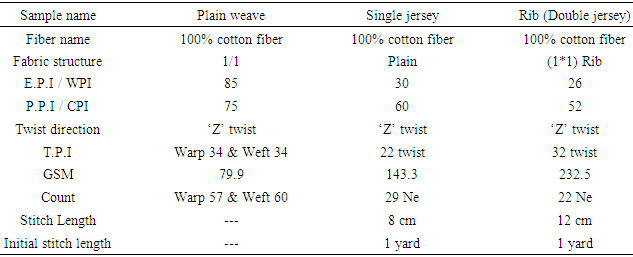 Color fastness to Dry Rubbing
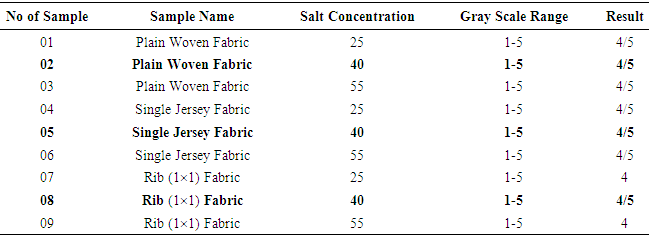
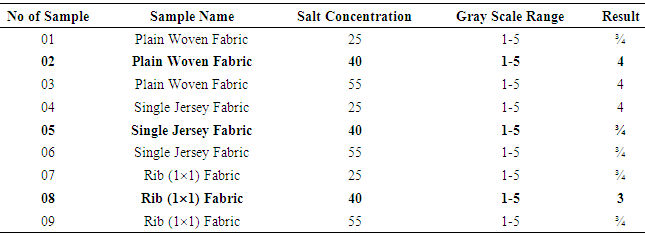
Color fastness to Dry RubbingTable 2. Dry Rubbing Fastness results
 |
| |
|
 | Figure 1. Plain Woven Fabric Dry rubbing fastness |
 | Figure 2. Single Jersey Fabric Dry rubbing fastness |
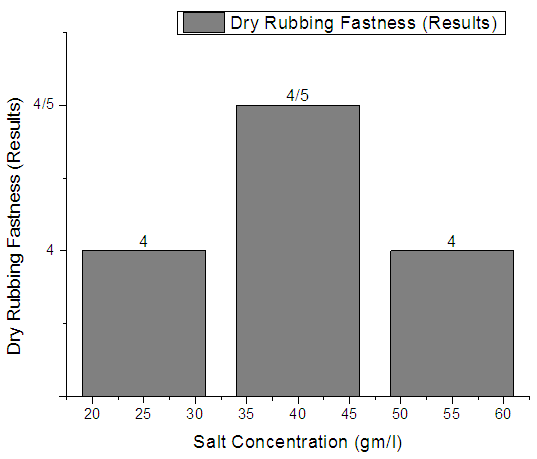 | Figure 3. Rib  Fabric Dry rubbing fastness Fabric Dry rubbing fastness |
From Figure 1, Figure 2, Figure 3 we have seen actual amount of salt dyed sample was proper shade, less amount of salt give light and high amount of salt give deep shade. We have also seen color fastness to Rubbing test result give all sample approximately same. So we can say salt amount do not influence fastness property. Color fastness to Wet RubbingTable 3. Wet Rubbing Fastness results
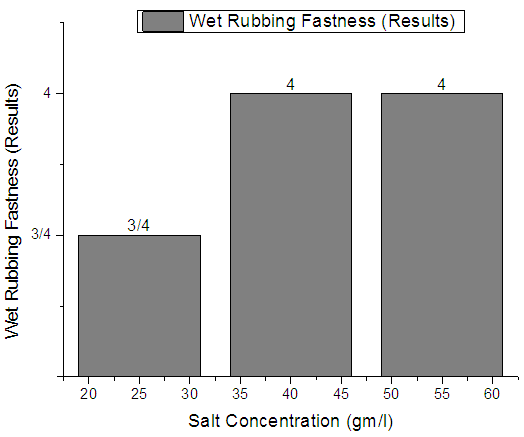
 |
| |
|
 | Figure 4. Plain Woven Fabric Wet rubbing fastness |
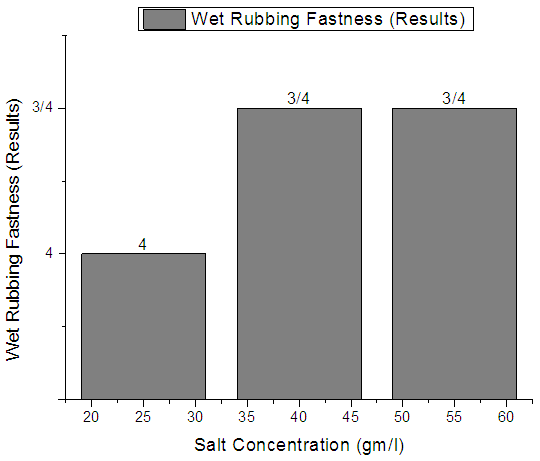 | Figure 5. Single Jersey Fabric Wet rubbing fastness |
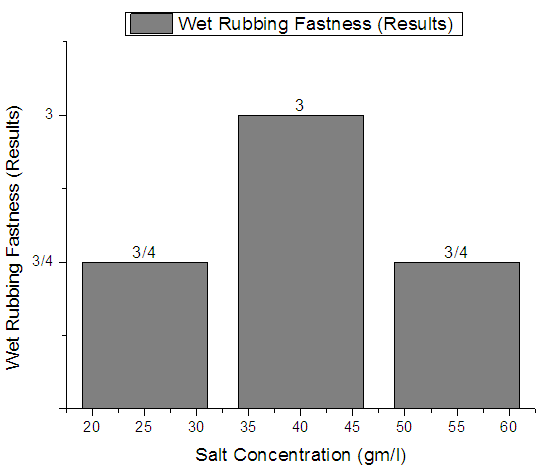 | Figure 6. Rib  Fabric Dry rubbing fastness Fabric Dry rubbing fastness |
From Figure 4, Figure 5, Figure 6 we have seen actual amount of salt dyed sample was proper shade, less amount of salt give light and high amount of salt give deep shade. We have also seen color fastness to Rubbing test result give all sample approximately same. So we can say salt amount do not influence fastness property. Color fastness to washTable 4. Wash Fastness results
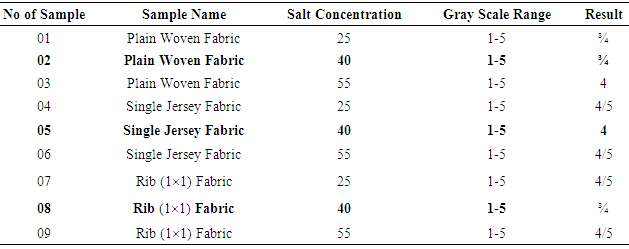 |
| |
|
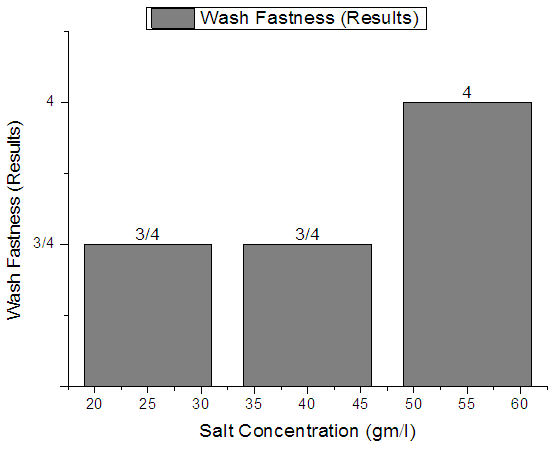 | Figure 7. Plain Woven Fabric Wash fastness |
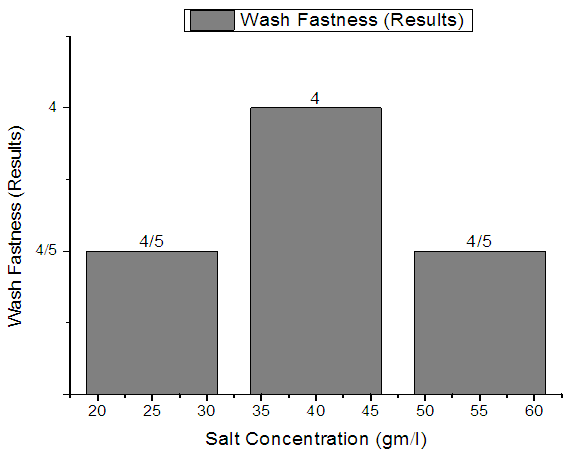 | Figure 8. Single Jersey Fabric Wash fastness |
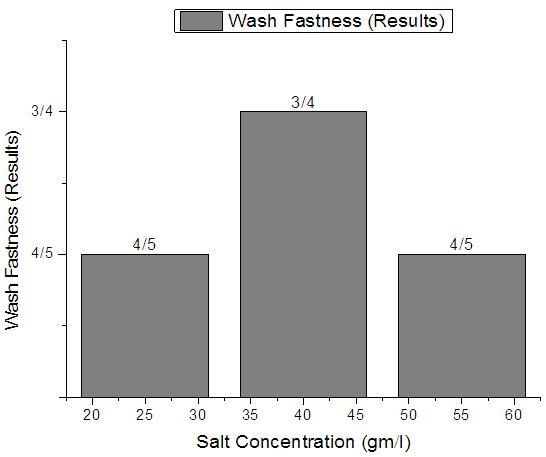 | Figure 9. Rib  Fabric Wash fastness Fabric Wash fastness |
From Figure 7, Figure 8, Figure 9 we have seen actual amount of salt dyed sample was proper shade, less amount of salt give light and high amount of salt give deep shade. We have also seen color fastness to Wash test result give all sample approximately same. So we can say salt amount do not influence fastness property.
4. Conclusions
The pretreatment, is applied through pad-dry-cure process, brings about some salt change in the treated fabric. Fastness properties are adequate and quite comparable with conventionally dyed samples. The rubbing fastness of the dyed fabric change slightly. When dyeing the modified substrates, reactive Dyes can be much more efficiently exhausted and fixed onto Cellulosic fabric under neutral condition in the variation of Salt concentration. The modified Dyeing don’t suffer either from a significant drop in wash fastness and perspiration fastness. There has significant effect on dyed fabric appearance and quality for the variation use of different concentration of salt with reactive dyes. That was showed good all fastness properties for different types of structural fabric on salt concentration.
ACKNOWLEDGEMENTS
This research paper is viable to write down even though the help and corroboration each Author’s. We would really like to babble precise gratitude and honor toward the member of Micro Fiber organization for their type co-operation, encouragement, the spur which assist us in finishing touch of this research paper.
References
| [1] | Nithyanandan, R. and M. Subramanian, Salt & Alkali Free Reactive Dyeing, p. 1-5. |
| [2] | http://www.chromogenix.com/technology/basic-principles/chromogenic-substrates.aspx. |
| [3] | Aspland, J.R., Textile Dyeing and Coloration. Textile Hub 2006. 1(3): p. 265-274. |
| [4] | Acharya and e.a. Sanjit, Chemical cationization of cotton fabric for improved dye uptake. Cellulose, 2014. 21(6): p. 4693-4706. |
| [5] | http://0x9.me/SCcgG. |
| [6] | Iqbal and e.a. Naseem, Asymmetric bioreduction of activated carbon–carbon double bonds using Shewanella yellow enzyme (SYE-4) as novel enoate reductase. Tetrahedron, 2012. 68(37): p. 7619-7623. |
| [7] | Broadbent and A.D, Basic Principle of Textile Coloration. A.S.T.M, 2001. 1(2): p. 337. |
| [8] | http://0x9.me/xSRFn. |
| [9] | https://www.britannica.com/technology/dye. |
| [10] | Farook, Y., Textile Dyeing and Printing Technology. textile Today, 1997. 2(3): p. 178-190. |



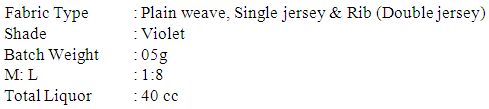 Recipe for salt requirement (15% less)
Recipe for salt requirement (15% less)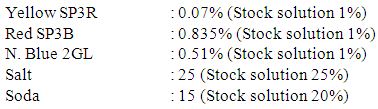 Recipe for actual salt requirement
Recipe for actual salt requirement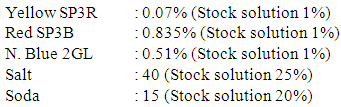 Recipe for salt requirement (15% addition)
Recipe for salt requirement (15% addition) General dyes calculation formula
General dyes calculation formula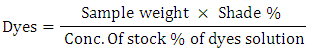
 Equation 1General chemicals calculation formula
Equation 1General chemicals calculation formula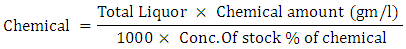
 Equation 2
Equation 2 Method of testing Color fastness to RubbingPrincipleThis test is designed to determine the degree of color which may be transferred from the surface of a colored fabric to a specific test cloth for rubbing (dry + wet).EquipmentØ Crock meter, Cotton rubbing cotton, Grey scale, Stop watch & Color matching cabinetSize of fabric4×5 cm two pieces of sample (one warp direction/wale direction & another weft/course direction).Test procedureØ Lock the test specimen onto the base of the crock meter.Ø Using the spinal spring clip, set 5cm5cm of the white cotton fabric to the finger of the crock meter.Ø Lower the covered finger on the test sample.Ø Turn hand crank at the rate of one turn per second (1010 sec).Ø Remove the white rubbing test cloth and evaluate with grey scale.EvaluationEvaluation the contrast between the treated and untreated white rubbing cloth with grey scale and rated 1-5.Method of testing Color fastness to washColor fastness to wash is very important for lab-dip. There are varieties of testing procedure, because-Ø The methods depend on the use of dyed goods.The degree of fading and staining of dyed goods for washing depends upon the following factors:Ø Temperature range may be from 40°C-95°C.Ø The type and amount of detergent added to the washing bath. In many testing procedure a standard detergent is used. The washing liquor to goods ratio is 50:01.Principle of wash fastnessA specimen (lab-dip) in contact with specified adjacent fabric or fabric or fabric is laundered, rinsed and dried. The specimen/composite sample is treated under appropriate condition in a chemical bath for short time. The abrasive action is accomplished by the use of a liquor ratio and an appropriate number of steel balls. The change in color of the specimen (dyed sample) and the staining of the adjacent fabric is assessed by recommended Grey scale (1-5) [10].Apparatus and materialsØ Wash –wheel with a thermostatically controlled water bath and rating speed of (40±2) rpm.Ø Stainless steel container (capacity 55±50 ml)Ø Stainless steel ball (dia = 0.6cm, weight = 1 gm)Ø SDC, Multifibre fabric (Acetate, cotton, nylon, polyester, acrylic, wool)Ø Thermometer, Sewing machine, Dryer, Color matching cabinet and ISO Grey Scales.ReagentsØ Sodium perborate (NaBo2.H2O.3H2O)Ø ECE without brightening agent & Distilled water Test specimenTest specimen cut a sample of dyed goods 10*4cm and sew it with same size multifibre fabric. This is the composite test sample. Evaluation of wash fastnessCompare the contrast between the treated and untreated sample with Grey Scale for changing color of dyed sample and staining of adjacent fabric in a color matching cabinet. Numerical rating for color changing is the shade and staining to adjacent fabric. Number of method used.Assessment of color fastness
Method of testing Color fastness to RubbingPrincipleThis test is designed to determine the degree of color which may be transferred from the surface of a colored fabric to a specific test cloth for rubbing (dry + wet).EquipmentØ Crock meter, Cotton rubbing cotton, Grey scale, Stop watch & Color matching cabinetSize of fabric4×5 cm two pieces of sample (one warp direction/wale direction & another weft/course direction).Test procedureØ Lock the test specimen onto the base of the crock meter.Ø Using the spinal spring clip, set 5cm5cm of the white cotton fabric to the finger of the crock meter.Ø Lower the covered finger on the test sample.Ø Turn hand crank at the rate of one turn per second (1010 sec).Ø Remove the white rubbing test cloth and evaluate with grey scale.EvaluationEvaluation the contrast between the treated and untreated white rubbing cloth with grey scale and rated 1-5.Method of testing Color fastness to washColor fastness to wash is very important for lab-dip. There are varieties of testing procedure, because-Ø The methods depend on the use of dyed goods.The degree of fading and staining of dyed goods for washing depends upon the following factors:Ø Temperature range may be from 40°C-95°C.Ø The type and amount of detergent added to the washing bath. In many testing procedure a standard detergent is used. The washing liquor to goods ratio is 50:01.Principle of wash fastnessA specimen (lab-dip) in contact with specified adjacent fabric or fabric or fabric is laundered, rinsed and dried. The specimen/composite sample is treated under appropriate condition in a chemical bath for short time. The abrasive action is accomplished by the use of a liquor ratio and an appropriate number of steel balls. The change in color of the specimen (dyed sample) and the staining of the adjacent fabric is assessed by recommended Grey scale (1-5) [10].Apparatus and materialsØ Wash –wheel with a thermostatically controlled water bath and rating speed of (40±2) rpm.Ø Stainless steel container (capacity 55±50 ml)Ø Stainless steel ball (dia = 0.6cm, weight = 1 gm)Ø SDC, Multifibre fabric (Acetate, cotton, nylon, polyester, acrylic, wool)Ø Thermometer, Sewing machine, Dryer, Color matching cabinet and ISO Grey Scales.ReagentsØ Sodium perborate (NaBo2.H2O.3H2O)Ø ECE without brightening agent & Distilled water Test specimenTest specimen cut a sample of dyed goods 10*4cm and sew it with same size multifibre fabric. This is the composite test sample. Evaluation of wash fastnessCompare the contrast between the treated and untreated sample with Grey Scale for changing color of dyed sample and staining of adjacent fabric in a color matching cabinet. Numerical rating for color changing is the shade and staining to adjacent fabric. Number of method used.Assessment of color fastness  Color fastness to Dry Rubbing
Color fastness to Dry Rubbing


 Fabric Dry rubbing fastness
Fabric Dry rubbing fastness


 Fabric Dry rubbing fastness
Fabric Dry rubbing fastness


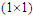 Fabric Wash fastness
Fabric Wash fastness Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML


