-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Textile Science
p-ISSN: 2325-0119 e-ISSN: 2325-0100
2016; 5(6): 132-140
doi:10.5923/j.textile.20160506.02

Review of Renewable Biosorbent from Coir Pith Waste for Textile Effluent Treatment
Muksit Ahamed Chowdhury1, Konica Jannat Fatema2
1Assistant Professor, Department of Textile Engineering, Ahsanullah University of Science and Technology, Dhaka, Bangladesh
2Senior Scientific Officer, Chemistry Division, Bangladesh Atomic Energy Center, Dhaka, Bangladesh
Correspondence to: Muksit Ahamed Chowdhury, Assistant Professor, Department of Textile Engineering, Ahsanullah University of Science and Technology, Dhaka, Bangladesh.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Biosorption is an innovative technology aimed at the removal of toxic metals from effluent utilizing abundantly available agricultural waste biomaterials. Among several agricultural wastes studied as biosorbents for effluent treatment, coir pith has been of great importance for the removal of diverse type of pollutants from effluent water. As an agricultural waste, coir pith has gained wide attention as effective biosorbents due to the significant adsorption potential for the removal of various aquatic pollutants. Cost-effectiveness, abundant availability and renewability makes them as an economical alternative for water treatment and waste remediation. In this review, the perspective of coir pith based biosorbents for textile effluent treatment from vast literature has been compiled and their adsorption capacities for various types of as available in the literature are presented.
Keywords: Coir pith, Activated carbon, Biosortion, Agricultural waste materials, Effluent treatment
Cite this paper: Muksit Ahamed Chowdhury, Konica Jannat Fatema, Review of Renewable Biosorbent from Coir Pith Waste for Textile Effluent Treatment, International Journal of Textile Science, Vol. 5 No. 6, 2016, pp. 132-140. doi: 10.5923/j.textile.20160506.02.
Article Outline
1. Introduction
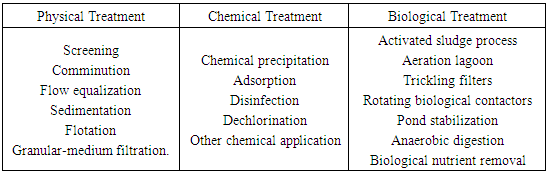
- Textile industries are large industrial consumers of waters as well as producers of waste waters. With the increased demand for textile products, the textile industry and its waste waters have been increasing proportionally, making it one of the main sources of severe pollution problems worldwide [1]. The chemical reagents used in textile processing are very diverse in chemical composition, ranging from inorganic compounds to polymers and organic products [2]. Textile waste water may include many types of dyes, detergents, insecticides, pesticides, grease and oils, sulfide compounds, solvents, heavy metals, inorganic salts and fibers, in amounts depending on the processing system [3]. Colored matter discharged from textile industries is not only aesthetically displeasing, but also affect aquatic ecosystems by inhibiting sunlight penetration and posing certain hazards and environmental problems. Many types of dye are used in the textile industry such as direct, reactive, acid and basic and disperse dyes, which can be toxic to some microorganisms and may cause direct destruction or inhibition of their metabolism. Color removal of effluent from the textile dyeing and finishing operation is becoming important because of aesthetic as well as environmental concerns. It is reported that there are over 100,000 commercially available dyes and more than 7.5 X 105 metric tons are produced all over the world every year and most of them are completely resistant to biodegradation processes [4, 5]. Many of these dye wastes are toxic and even carcinogenic [6] and this poses a serious hazard to aquatic living organisms. It is estimated that 10–15% of the dye is lost in the effluent during manufacturing and processing operations [7]. As a result, many governments have established environmental restrictions with regard to the quality of colored effluents and have forced dye houses to decolorise their effluents before discharging.Wastewaters containing dyes are difficult to remove because of their inert properties. The conventional methodologies for the removal of dyes such as – adsorption, adsorption on activated carbons, coagulation, flocculation, reverse osmosis, activated sludge, bacterial action, chemical oxidation, ozonation, and physical methods like membrane filtration, ion exchange and electrochemical techniques are either expensive or inadequate because of their stability towards light, oxidising agents and resistance towards aerobic digestion, besides generating sludge with disposal problem [8-11]. Different treatment processes available to textile effluent are given in Table 1 [12].
|
2. Coir Pith
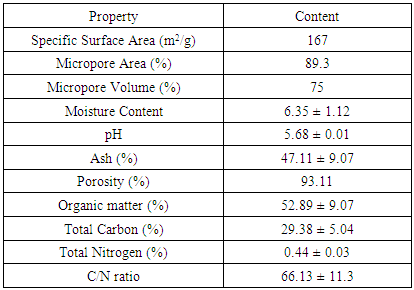
- Coir pith, a waste product obtained during the extraction of coir fibre from coconut husk, is very light, highly compressible and highly hygroscopic. A cross-section of a coconut is illustrated in Figure 1. Coconut, a common fruit produced abundantly in different countries like Indonesia, Philippine, India, Thailand, Malaysia and Bangladesh [15]. Coconut husk is the mesocarp of coconut and a coconut consists of 33–35% of husk. Husks are composed of 70% pith and 30% fiber on a dry weight basis. Raw coir pith consists of 35.0% cellulose, 25.2% lignin, 7.5% pentosans, 1.8% fats and resins, 8.7% ash content, 11.9% moisture content and 10.6% other substances. Physical and chemical characteristics of coir pith are given in Table 2 [18, 19].
 | Figure 1. Cross-section of a coconut |
|
3. Preparation of Absorbent
- The general schematic diagram for preparation of powdered activated carbon from agricultural solid waste, i.e. coir pith has been given in Fig. 2. At first coir pith need to wash properly to remove any mud, debris, etc., dried and then ground to a particle size of 2 mm for the further process of carbonization. The waste materials are generally carbonized in a conventional electric furnace by a two stage carbonization process under optimized conditions. Char was prepared by keeping the biomass waste in covered stainless steel vessels and carbonized at 400°C for 30 min in a muffle furnace followed by chemical activation by impregnating with ZnCl2 for 24 h at a 1.75 ZnCl2/char ratio and dried at 100 ± 5°C. The dried and impregnated char was carbonized at 650°C for 15 min. Before utilization, the activated product need to be treated with 1:1 HCl for the removal of impregnating salt, followed by washing with hot distilled water for the removal of chlorides and acidity. Finally the activated product dried properly to obtain powder activated carbon [28].
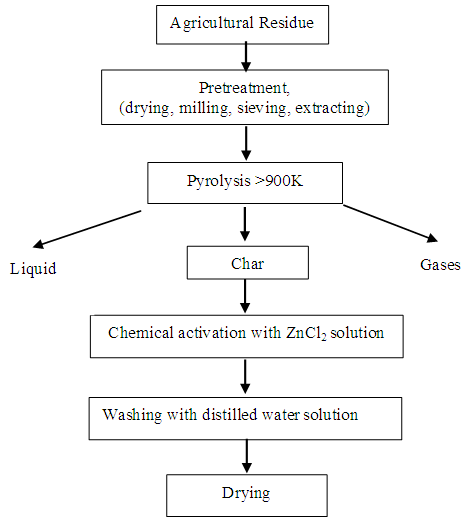 | Figure 2. Process flow diagram of activated carbon preparation from agricultural residue [29] |
4. Application of Coir Pith for Removal of Dyes from Effluent Water
- The worldwide high level of dyes production and their extensive use in dyeing textiles generate colored wastewaters which cause severe water pollution. The colored dye effluents are generally considered to be highly toxic to the aquatic biota and also cause many health related problems such as allergy, dermatitis, skin irritation, cancer, and mutations in humans [30]. Thus, the removal of dyes from effluents before they are mixed up with natural water bodies is important. Coir pith-based biosorbents have been widely explored for the removal of different classes of dyes from aqueous solutions.
4.1. By Raw Coir Pith
- Removal of acid dyes (acid yellow 99) was carried out using ‘waste’ coir pith as adsorbent by Khan et al. Adsorption capacities of coir pith for acid yellow was found to be 442.13 mg/g. The adsorption process is found to be a function of pH of the solution, the optimum pH value being 2.0 and it is not affected by incubation temperature. The process was found very fast initially and both chemisorptions and physisorptions take place simultaneously [8].
4.2. By Activated Carbon Prepared from Coir Pith
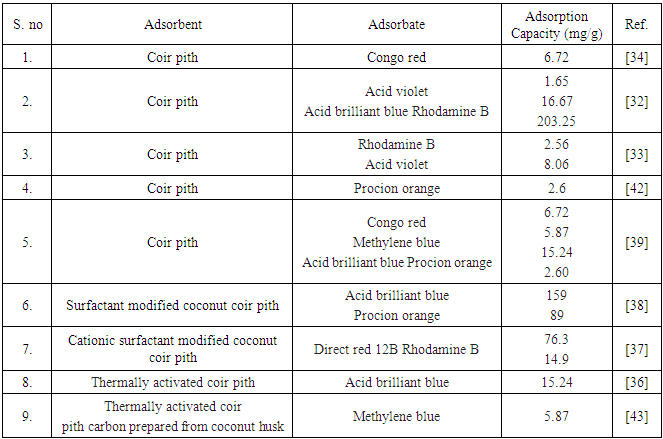
- As previously mentioned, coir pith is rich in lignocellulosic content; these materials can be used for the preparation of activated carbon. Activated carbon is a crude form of graphite with a random or amorphous structure, which is highly porous, exhibiting a broad range of pore sizes, from visible cracks, crevices and slits of molecular dimensions [31]. However, activated carbons are expensive due to their regeneration and reactivation procedures. In recent years, growing research interest in the production of carbon based has been focused on agricultural residues. Activated carbon prepared from coir pith was successfully used for removal of dyes. The removal efficiency of three highly used reactive dyes (C.I. Reactive Orange 12, C.I. Reactive Red 2 & C.I. Reactive Blue 4) in the textile industry was investigated by Santhy et al. using activated carbon prepared from coir pith [31]. The results showed that activated carbon prepared from coir pith was a suitable adsorbent for removal of reactive dyes from both synthetic and textile effluent. Adsorption followed the first-order rate expression and the adsorption increased with an increase of contact time and carbon dose and decreased with an increase in solute concentration. Removal of dyes was higher at the acidic pH range. The exhausted carbon could be completely regenerated and put to repeated use by elution with 1.0 M NaOH. The coir pith activated carbon was not only effective in removal of color but also significantly reduced COD levels of the textile wastewater.Using ‘waste’ coir pith as adsorbent, removal of acid dyes (acid violet and acid brilliant blue) and basic dyes (rhodamine B and methylene blue) were carried out Namasivayam et al [32]. Adsorption capacities of coir pith for acid violet, acid brilliant blue and rhodamine B were found to be 1.65, 16.67 and 203.25 mg/g, respectively. At alkaline pH, lower adsorption of acid violet and acid brilliant blue was observed and it was attributed to the presence of excess OH− ions competing with dye anion for the adsorption sites, whereas a reverse trend was observed for methylene blue. Lower adsorption of methylene blue at highly acidic pH was attributed to the presence of excess H+ ions competing with the dye cation for the adsorption sites. But rhodamine B showed a different trend, where the adsorption was maximum (85%) at acidic pH (3.03) and decreased to 26.6% with further increase in pH (11.03). From desorption experiments, it was confirmed that for the dyes, acid brilliant blue and methylene blue, the major mode of adsorption was ion exchange, whereas acid violet showed physical adsorption. For rhodamine B, chemisorption was suggested to be the major mode of adsorption.The suitability of coir pith carbon was also examined for the adsorption of rhodamine-B and acid violet by Namasivayam et al [33]. The adsorption capacity was found to be 2.56 mg and 8.06 mg dye per g of the adsorbent for rhodamine-B and acid violet, respectively. Acidic pH was found favorable for the adsorption of acid violet and alkaline pH for rhodamine-B. The desorption studies showed that the major modes of adsorption might be ion exchange and physical adsorption.Coir pith carbon has also been employed for the adsorption of Congo red [34]. The adsorption capacity was found to be 6.72 mg per g of the adsorbent. Acidic pH was favourable for the adsorption of Congo Red. Complete removal of the dye can be achieved using an appropriate dosage of the adsorbent and pH for wastewaters. Desorption studies suggest that chemisorption might be the major mode of adsorption.Kavitha et al. investigate the feasibility of thermally activated coir pith carbon prepared from coconut husk for the removal of methylene blue [35]. The adsorption capacity was found to be 5.87 mg/g by Langmuir isotherm for the adsorbent particle size of 250–500 µm. The equilibrium time was found to be 30 and 60 min for 10 and 20 mg/l and 100 min for 30, 40 mg/l dye concentrations, respectively. At pH 6.9, a maximum removal of 97% was obtained for an adsorbent dose of 100 mg/50 ml and 100% removal was obtained for an adsorbent dose of 600 mg/50 ml of 10 mg/l dye concentration. Based on pH and desorption studies, the authors concluded that chemisorption might be the major mode of the adsorption process. The same researchers also studied the potential feasibilities of thermally activated coir pith carbon for the removal of acid brilliant blue dye from aqueous solution [36]. At pH 2 maximum removals for acid brilliant blue with carbon was observed, when the surface was positively charged with excess protons in solution. The adsorption capacity was found to be 15.24 mg/g by Langmuir isotherm. The pH effect and desorption studies suggested that chemisorption might be the major mode of the adsorption process.
4.3. By Surface Modified Coir Pith
- The adsorption capacity of coir pith toward anionic dyes was found to be very low. To enhance the sorption capacity towards anionic contaminants, the surface modification of the coir pith was performed using a cationic surfactant, hexadecyl trimethyl ammonium bromide [37]. Adsorption of two anionic dyes, acid brilliant blue (acid dye) and procion orange (reactive dye), were studied. It was found that modified coir pith yielded adsorption capacities of 159 and 89 mg/g for acid brilliant blue and procion orange, respectively. Dye adsorption increased with increased temperature, indicating that the process was endothermic. The main mechanisms involved in the process were suggested as ion exchange and chemisorption for the uptake of dyes.
|
4.4. Mechanism of Absorption of Dye by Coir Pith Carbon
- The surface chemistry of carbon is determined to a large extent by the number and the nature of the surface functional groups or complexes. Mineral admixtures present in activated carbons can also influence the surface chemistry of carbons. Carbon–oxygen surface compounds are by far the most important in influencing surface reactions, surface behaviour, hydrophilicity and electrical and catalytic properties of carbons, cationic or anionic exchange capacities have been observed for carbons depending on surface functionality [40]. In one study, adsorption interactions of dyes, phenol and chlorophenols onto coir pith carbon from aqueous solution were investigated using FTIR spectroscopy, Scanning Electron Microscope (SEM), and X-ray diffraction techniques by Namasivayam and Kavitha [41]. IR studies indicate the participation of some of the surface functional groups in the adsorption mechanism and SEM studies visualized the formation of the molecular cloud of the dye/phenol over the surface. The XRD data of the adsorbate loaded carbon had evidence of the crystalline nature of carbon changing in amorphous nature after adsorption which suggested that the dye and phenol molecules diffuse into micro-pores and macro-pores of the adsorbent and adsorb mostly by chemisorptions with altering the structure of the carbon, as a result of the adsorption reaction.
5. Application of Coir Pith for Removal of Toxic Metal Ions from Water
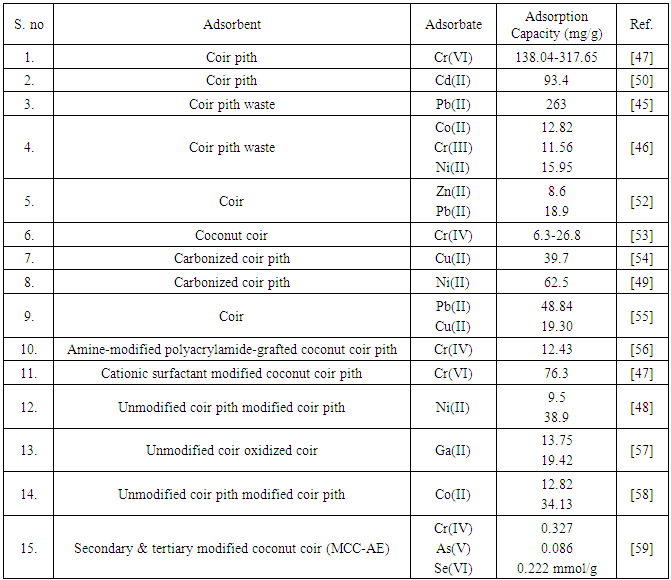
- The presence of elevated concentrations of toxic metal ions in the water body represents a major risk to plants, animals and human health. Various toxic metal ions are discharged into the environment through various industrial activities, constituting one of the major causes of environmental pollution. One of the important features that distinguish heavy metals from other pollutants is that the former are not biodegradable. Once metal ions enter the environment, their chemical form largely determines their potential toxicity. Coir pith has been extensively investigated as adsorbents for the removal of diverse metal ions from wastewater by different researchers.Coir pith waste was used for the adsorptive removal of Pb(II) from aqueous solution by Kadirvelu and Namasivayam [44]. The adsorption capacity was reported to be 263 mg/g for Pb(II) removal which was found to increase with increasing pH from 2 to 4 and remained constant up to pH 10. Parab et al. studied coir pith as adsorbent for Co(II), Cr(III) and Ni(II) adsorption in both single and multi-component systems [45]. The maximum adsorption capacity of coir pith was found to be 12.82, 11.56 and 15.95 mg/g for cobalt, chromium and nickel, respectively. Optimum pH values for maximum metal ion adsorption were found as 4.3 for cobalt, 3.3 for chromium and 5.3 for nickel. In a study Suksabye et al. investigated the use of coir pith for the removal of hexavalent chromium from electroplating wastewater [46]. The maximum removal (99.99%) was obtained at 2% (w/v) dosage, particle size b75 µm, at initial Cr(VI) concentration 1647 mg/L, system pH 2, and an equilibrium time of 18 h. The maximum adsorption capacity of coir pith for Cr(VI) at 15, 30, 45 and 60°C was 138.04, 197.23, 262.89 and 317.65 mg Cr(VI)/g coir pith, respectively.Namasivayam and Sureshkumar [47] investigated the feasibility of coconut coir pith as biosorbent for the removal of Cr(VI) after modification with a cationic surfactant, hexadecyl trimethyl ammonium bromide. Optimum pH for Cr(VI) adsorption was found to be 2.0. Reduction of Cr(VI) to Cr(III) occurred to a slight extent during the removal. The adsorption capacity of the biosorbent was found to be 76.3 mg/g. The removal of nickel from the waste water was investigated by Ewecharoen et al. [48]. The optimum condition for nickel removal by coir pith in a batch system was found solution pH of 4–7, adsorbent dosage of 5% (w/v), an equilibrium contact time of 10 min and a temperature of 30°C. The maximum uptake of nickel by coir pith and modified coir pith were 9.5 and 38.9 mg/g, respectively. Sodium hydroxide increased metal binding site (free O-) of modified coir pith. It was found that lignin and holo-cellulose were the main components in coir pith that played a major role in nickel adsorption. The IR spectrum study revealed that the main functional groups which were involved in the nickel adsorption were hydroxyl and methoxy groups.Kadirvelu et al. examined the use of carbonized coir pith for the adsorption of Ni(II) from aqueous solution [49]. It was reported that a decrease in the carbon concentration with constant Ni concentration, or an increase in the Ni concentration with constant carbon concentration resulted in a higher nickel uptake per unit weight of carbon. The adsorption capacity was found 62.5 mg/g at an initial pH of 5.0 at 30°C for the particle size 250–500 µm. The adsorption of Ni increased with pH from 2 to 7 and remained constant up to 10. The recovery of Ni(II) after adsorption was carried out by treatment of the Ni loaded carbon with HCl. Desorption studies confirmed that adsorption mechanism was ion exchange.Kadirvelu and Namasivayam developed activated carbon from coir pith for the adsorption of Cd(II) from aqueous solution [50]. The adsorption capacity was found 93.4 mg/g at an initial pH of 5.0 at 30°C for the particle size 250–500 mm. The percent removal increased with pH from 2 to 4 and remained constant up to pH 10. The maximum percentage recovery of Cd(II) was observed as 100% with 0.075 M HCl solution, showing that ion exchange was involved in the adsorption process.Carbonized coir pith has also been used for Cu(II) removal from aqueous solutions by Namasivayam and Kadirvelu [51]. The percent removal increased from 50 to 90 with the increase of pH from 2.0 to 4.0 and remained constant up to pH 10.0 for Cu(II) concentration of 20 mg/L. The adsorption capacity of carbonized coir pith was found to be 39.7 mg/g at pH 4.2 for the particle size 250 to 500 µm. The percent recoveries of Cu(II) for carbonized coir pith were 60.0, 74.0, 93.0, 100.0 and 100.0 by 0.025, 0.05, 0.10, 0.15 and 0.2 M HCl, respectively, suggesting that ion exchange was involved in the sorption process. A summary of the adsorption capacity of coir pith based biosorbents for different metal ions has been presented in Table 4.
|
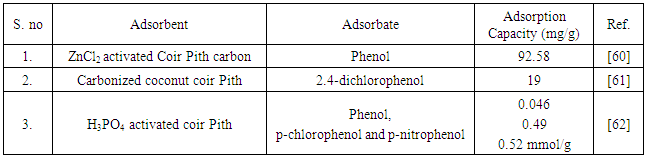
6. Application of Coir Pith for Removal of Phenolic Pollutant from Water
- Though textile effluent contributes a little phenol pollution, phenols are generally considered to be one of the important organic pollutants discharged into the environment. The discharge of effluent containing phenolic pollutants from various industries into natural water bodies is an ongoing and serious threat to human health and natural water quality. The ubiquitous nature of phenols, their toxicity even in trace amounts and the stricter environmental regulations make it necessary to develop processes for the removal of phenols from wastewaters. Coir pith based biosorbents have also been investigated for the removal of phenolic pollutants from water and wastewater. Table 5 illustrates the summary of the adsorption capacities of coir pith base biosorbents for the removal of various phenols from water.
|
7. Bioregenaration
- Bioregeneration is the renewing of activated carbon by microbial activities. Bioregeneration can be achieved either by mixing bacteria with saturated activated carbon in off line [62] or it can be achieved in the course of biological treatments as in the case of PACT or BAC systems. Bioregeneration can be optimized by varying the nature of microorganisms, the environmental conditions and the loading on activated carbon [63].Bioregeneration can only occur with compounds that readily desorb. Hence, bioregeneration is controlled by the reversibility of adsorption [62]. Effective bioregeneration processes depend on numerous factors including reversibility of adsorption, the presence of microbial organisms capable of metabolizing the adsorbate, the settings of optimal microbial growth conditions including nutrients (nitrogen, phosphorus, sulfur, etc.) temperature, dissolved oxygen, etc. and optimization of microbial and adsorbate concentration ratios [31].
8. Conclusions and Future Perspective
- To date, rapidly changing technologies, population growth, industrial products and practices are increasing worldwide, driving towards the overwhelming environment pollutions, ecological imbalance, upsetting of biological processes and threatening of the public health. Recently coir pith based biosorbents are extensively studied for the removal of diverse type of pollutants from wastewater. In this review, uses of coir pith for the adsorption of toxic dyes and metal ions from effluent water were summarized based on a substantial number of relevant published articles. The use of coir pith as a biosorbents for removing various types of dyes from effluent water offers many attractive features such as the outstanding adsorption capacity for many pollutants and the fact that these materials are low-cost, non-toxic and biocompatible. Although the amount of available literature data for the application of coir pith for adsorption of dyes and metal ions from aqueous solution is increasing at a remarkable pace, there are still several gaps which need more attention, such as enhancement of biosorption capacity through modification of biosorbent, assessment of biosorbents under multi-component dyes and pollutants, mechanistic modelling to correctly understand the sorption mechanisms, investigation of these materials with real industrial effluents, recovery of dyes, regeneration studies and continuous flow studies. There is a particular need for future studies to verify the performance of the coir pith as low-cost adsorbents at the pilot plant scale. There is a great need for additional research concerning how to further process or disposed of coir pith after it has been used to collect dyes, some of which may be toxic, slow to biodegrade, or subjected to leaching [33]. Despite various challenges has been identified and clarified, a widespread and greatly the progress of in this area can be expected in the future.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML