-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Textile Science
p-ISSN: 2325-0119 e-ISSN: 2325-0100
2016; 5(3): 60-65
doi:10.5923/j.textile.20160503.03

Enhancing the Dyability of Linen Fabrics to Metal Complex Dye Using Nitrogenous Additives
El-Zairy W. M. R.
Textile Printing, Dyeing and Finishing Dept., Faculty of Applied Arts, Helwan University, Giza, Egypt
Correspondence to: El-Zairy W. M. R., Textile Printing, Dyeing and Finishing Dept., Faculty of Applied Arts, Helwan University, Giza, Egypt.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Chemical modification of linen fabric via cationization with chitosan as well as Quat-188 has been recently studied. Desirable properties are imparted to linen fabric which render to the patter cationic in nature having a persistent positive charges and can thereby lead to the formation of ionic bonds salt linkages with negatively charged has no affinity for cellulosic fibers. Heriene, the former anionic dyes-class can successfully be dyed cationized linen fabric using no salt dyeing method resulting higher dye fixation as well as deep color yield (K/S), which is unequivocally due to electrostatic attraction of the dye molecules towards cationic sites in cationizated linen fabrics. The color fastness ratings of the dyed aminized linen fabrics recoverded very good results which is refer to the highly dye fixation on the modified substrate.
Keywords: Linen, Metal complex dyes, Dyeing, Chitosan, Quat-188, Cationization
Cite this paper: El-Zairy W. M. R., Enhancing the Dyability of Linen Fabrics to Metal Complex Dye Using Nitrogenous Additives, International Journal of Textile Science, Vol. 5 No. 3, 2016, pp. 60-65. doi: 10.5923/j.textile.20160503.03.
Article Outline
1. Introduction
- Linen is the term used for fibers received from the flax plant. It is popular for garments and home textiles which is valued for its exceptional coolness and freshness in hot weather conditions as well as its natural touch and look [1-4]. Linen has a number of properties that makes it useful for various textile applications like home furnishings and apparels in which absorbency and drying properties are notable, and also absorbs significant amount of moisture while dries relatively fast compare to cotton. It is most suitable for use in hot season, as the fiber allows the heat to escape, leaving a cool effect [5-7]. Many studies investigated the bleaching efficiency of enzymatically scoured linen fabrics using a combined laccase-hyrogen peroxide process. Their pretreatment improves reactive and cationic dyeing performances of linen fabrics [8].Metal complex dyes represent an important dye-class for fibers contained amino groups in their chemical structure, e.g., wool, silk and nylon 66, hence these dyes are not suitable for dyeing and printing cellulose, e.g., flax yarns because of its little or no affinity for the late fibers [9].Attempts were made in the present work to find the most appropriate pretreatment for emulation for modifying linen fabric to impart special chemical properties into its cellulosic structure, taking in consideration the environmental, economic and quality dimensions for producing innovative modified effective cellulose product having reactive amino groups that capable for dyeing with 1:2 metal complex dyes in acid medium. Hence chitosan is the most suitable bio-finishing agent that used for this purpose to modify linen fabric by introducing positively charged, e.g. amino groups onto the cellulose, i.e. linen fabric structure which can be able to react with the aforesaid dye-class.
2. Experimental
2.1. Materials
- Linen fabric (weave mill-scoured and bleached linen 100%, 150 g/m2) was used. (All chem.s) were of laboratory grade chemicals. 3-chloro-2hydroxypropyl trimethyl ammonium chloride (65% aqueous solution under a commercial name Quat-188), which was kindly supplied by DOW Chemical Company, USA. Chitosan (medium molecular weight, deacetylation 82.9% Dalton, Vanson Inc. Co. USA) -Arkofix NZF (modified dihydroxy ethylene urea) Crosslinking agent formaldehyde free, by Clariant Co.. Tissocyl RC9 (combination of non-ionic surfactants Detergent and wetting agent) by Z&S Company, Germany. Magnesium chloride hexahydrate (MgCl2.H2O). Metal complex dye under commercial name ACIDOL YELLOW M-2GL (by BASF).
2.2. Cationization of Linen Fabrics
- The linen fabrics were cationized in presence of (2,4,5,6% o.w.cationic agent), (60 g/l) Arkofix NZF (crosslink DMDHEU), 5 g/l MgCl2.6H2O, using pad - dry - cure technique. The samples were padded twice- with pick up 70-80% in the previous solution then dried at 85°C for 3 min then cured at (120-160c) for (1-4) min.at pH (1-7), followed by thoroughly washing and drying.
2.3. Post Dyeing of Cationized Linen Fabrics using Metal Complex Dye
- The cationized linen fabrics were dyed in a microwave oven with the dye solution only and without no salts or additives. The treated fabrics were dyed in a bath containing 2% metal complex dye at L.R 1: 50. The microwave oven was as a sharp modle R-210B, with an 800-W output operating at frequency of 2550 MHz. The dyed fabrics were squeezed, washed with water until the rinse become clear then washed at 60 °C for 15 min in the presence of 5 g/l soap, rinsed thoroughly and then air dried. The duration of dyeing, microwave intensity and dye conc. were studied.
3. Tests and Analyses
- The color strength of dyed cotton samples were measured by using reflectance spectrophotometer model Data color Spectrophotometer SF600+Datacolor Company, U.S.A. The color strength expressed as K/S values was assessed by applying the Kubelka Munk equation K/S= (1- R) 2 /2R, Where K and S are the absorption and scattering coefficient respectively, and R is the reflectance of the dyed fabric. Nitrogen content of the cationized fabrics was determined according to kjeldahl method. Fastness properties of dyed samples were tested according to ISO standard methods. The specific tests were: ISOX12 (1987), color fastness to washing; and ISO 105-E04, color fastness to perspiration, as well as the dyed samples were subjected to tests, for fastness to light by AATCC test method 16-1993.
4. Result and Discussion
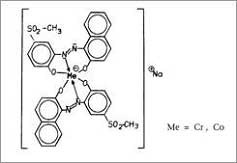
- Metal complex dyes or premetallised acid dyes, implies as their name one metal atom, commonly chromium or cobalt is complex with either one (1:1 metal complex dye) or two (1:2 metal complex dye) molecules of a typically monoazo dye that contain groups such as hydroxyl, carboxyl, or amino which are capable of co-ordinating with metal [10]. The dye ligands contain no ionizing groups which are free of strongly polar , ionic solubilising groups (i.e. –SO3H or –COOH) structure "a" 1:2 metal complex dye type of "Irgalan Brown Violet DL, when R = SO2CH3". 1:2 chelates of ligands which contain sulphonic acids to confer aqueous solubility, e.g. "b" are tribasic dyes and dyeable only in strongly acid medium, in such conditions, the dyes decompose into the 1:1 (zwitter ion) complex and the metal- free dye acid.In general, increases in negative charge decrease stability of acids.Adequate solubility in water is achieved by introducing highly polar but non-ionising groups, the two most valuable groups are the methyl sulphonyl (-SO2CH3) and the sulphamoyl (-SO2NH2).
 (a)
(a)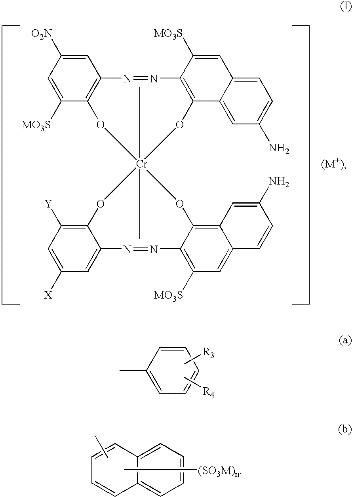 (b)The extent to which the 1:1 and 1:2 complexes are formed vary with the dye and conditions of the preparation; low solubility and low pH favour the formation of 1:1 complex, i.e. pH value of the dye- bath should be maintain about 2-2.4. On the other hand, the exhausion of 1:2 metal complex dyes is only slightly dependent on pH and there is a preference to dye them from weakly acid, neutral or even alkaline baths (pH 5-7) [11].Thus, the latter prementallised dye class, i.e. 1:2 metal complex type are suited for application on wool or polyamide when blended with cellulosic fibers, since they are dyed from a neutral dye bath in which a cellulose component is not damaged. There is not the case with 1:1 metal complex dyes, which need strong acid in the dye bath, and therefore cause damage of cellulose component of the blend during dyeing. Thus, it may be concluded that 1:1 metal complex dyes are not used on wool and nylon blended with cellulosic fibers [12]. Mechanism of formation an insoluble modified linen- 1:2 metal – complex dye interaction:The nature of interaction mechanism of 1:2 metal complex dye- cationized linen is similar to that of wool, as each of wool and cationised linen fabrics contained amino groups in fiber-chemical structure. The metal atom in both weakly and strongly 1:2 metal complex dyes is fully coordinated with the two dye lignads, as previously illustrated in their chemical structure, and, as a consequence, coordination of the chromium or cobalt dye ion with ligands such as amino or carboxyl groups in the fiber is not possible [13].Since a weakly polar 1:2 metal complex acid dye carries a single negative charge (due to the loss of four protons from the two component dye ligands), a monosulphonated 1:2 complex an overall negative charge of two and a disulphonated 1:2 complex an overall negative charge of three, then ion-ion interaction, operating between the dye anion and the protonated amino groups in the substrate i.e. Modified linen fabric, can be expected to contribute to dye-fiber substantively [14]. This electrostatic interaction can be expected to be more pronounced in the case of the strongly polar dyes which carry a comparatively greater and also localized negative charge than in the weakly polar dyes carrying a non-localized, single negative charge.The dyes are applied to substrates contained amino groups under weakly acidic to near- neutral pH conditions, however, and at such pH values the number of protonated amino groups in the modified linen fabrics is small, consequently, ion-ion interaction will be small, thus forces other than electrostatic will contribute to dye-fiber substantively. On the other hand, another approach is to enhance linen reactivity through chemical modification. This indeed, the main aim of the present work which is undertaken with a view to develop 1:2 metal complex dyeing process through environment of cationization of linen fabric via two different reagents, e.g., chitosan and quat-188 to impact the substrate desirable properties which render the patter cationic in nature, moreover, the present of cationized groups in the cellulose imparts also antimicrobial properties to linen fabrics [15].The nature of dye-fiber interaction mechanism has received little attention. It is assumed from the above discussion, that cationized linen could successfully be dyed with 1:2 metal-complex dye and this is unequivocally due to electrostatic attraction of dye molecules to the cationic sites in cationized linen which have persistent positive charges (NH3+) and can thereby lead to the formation of ionic bonds "salt linkages" with negative charg as found in 1:2 metal complex dye class. Farther, it is assumed that the aminized linen acts as an amphoteric ion exchanger and high adsorption in the neutral region of pH takes place because of high affinity of the dye which is this region may be accompanied depending on pH, partly by hydrogen and partly by sodium ions in order to maintain the fiber electrically neutral.As already pointed out, the aim of the current work was initiated to improve the dyeability of linen fabrics towards 1:2 premetalised dye, and the preferable selected dye was Acid yellow M-2GL.The obtained results along with the appropriate discussion, follows.
(b)The extent to which the 1:1 and 1:2 complexes are formed vary with the dye and conditions of the preparation; low solubility and low pH favour the formation of 1:1 complex, i.e. pH value of the dye- bath should be maintain about 2-2.4. On the other hand, the exhausion of 1:2 metal complex dyes is only slightly dependent on pH and there is a preference to dye them from weakly acid, neutral or even alkaline baths (pH 5-7) [11].Thus, the latter prementallised dye class, i.e. 1:2 metal complex type are suited for application on wool or polyamide when blended with cellulosic fibers, since they are dyed from a neutral dye bath in which a cellulose component is not damaged. There is not the case with 1:1 metal complex dyes, which need strong acid in the dye bath, and therefore cause damage of cellulose component of the blend during dyeing. Thus, it may be concluded that 1:1 metal complex dyes are not used on wool and nylon blended with cellulosic fibers [12]. Mechanism of formation an insoluble modified linen- 1:2 metal – complex dye interaction:The nature of interaction mechanism of 1:2 metal complex dye- cationized linen is similar to that of wool, as each of wool and cationised linen fabrics contained amino groups in fiber-chemical structure. The metal atom in both weakly and strongly 1:2 metal complex dyes is fully coordinated with the two dye lignads, as previously illustrated in their chemical structure, and, as a consequence, coordination of the chromium or cobalt dye ion with ligands such as amino or carboxyl groups in the fiber is not possible [13].Since a weakly polar 1:2 metal complex acid dye carries a single negative charge (due to the loss of four protons from the two component dye ligands), a monosulphonated 1:2 complex an overall negative charge of two and a disulphonated 1:2 complex an overall negative charge of three, then ion-ion interaction, operating between the dye anion and the protonated amino groups in the substrate i.e. Modified linen fabric, can be expected to contribute to dye-fiber substantively [14]. This electrostatic interaction can be expected to be more pronounced in the case of the strongly polar dyes which carry a comparatively greater and also localized negative charge than in the weakly polar dyes carrying a non-localized, single negative charge.The dyes are applied to substrates contained amino groups under weakly acidic to near- neutral pH conditions, however, and at such pH values the number of protonated amino groups in the modified linen fabrics is small, consequently, ion-ion interaction will be small, thus forces other than electrostatic will contribute to dye-fiber substantively. On the other hand, another approach is to enhance linen reactivity through chemical modification. This indeed, the main aim of the present work which is undertaken with a view to develop 1:2 metal complex dyeing process through environment of cationization of linen fabric via two different reagents, e.g., chitosan and quat-188 to impact the substrate desirable properties which render the patter cationic in nature, moreover, the present of cationized groups in the cellulose imparts also antimicrobial properties to linen fabrics [15].The nature of dye-fiber interaction mechanism has received little attention. It is assumed from the above discussion, that cationized linen could successfully be dyed with 1:2 metal-complex dye and this is unequivocally due to electrostatic attraction of dye molecules to the cationic sites in cationized linen which have persistent positive charges (NH3+) and can thereby lead to the formation of ionic bonds "salt linkages" with negative charg as found in 1:2 metal complex dye class. Farther, it is assumed that the aminized linen acts as an amphoteric ion exchanger and high adsorption in the neutral region of pH takes place because of high affinity of the dye which is this region may be accompanied depending on pH, partly by hydrogen and partly by sodium ions in order to maintain the fiber electrically neutral.As already pointed out, the aim of the current work was initiated to improve the dyeability of linen fabrics towards 1:2 premetalised dye, and the preferable selected dye was Acid yellow M-2GL.The obtained results along with the appropriate discussion, follows. 4.1. Effect of Type and Concentration of Cationising Agent
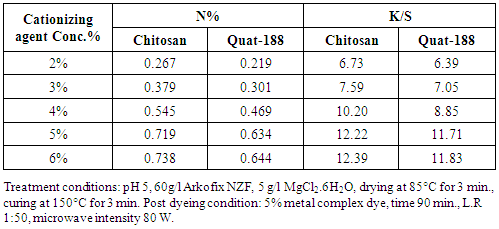
- The effect of the type and concentration of the cationising agents e.g., chitosan and Quat-188 was evaluated by measuring the nitrogen content and K/S of the modified fabrics at different concentrations (2,3,4,5,6 owf) of cationising agent along with other ingredients in the finishing formulations on the extent of modification and subsequent post dyeing of the linen fabrics. Table 1 shows the change in the nitrogen content, N%, of the treated fabrics in the absence and presence of certain cationising agent namely chitosan(beta(1,4)2(amino)2-deoxy-Dglycopyranose.) and Quat-188 (3-chloro-2hydroxy propyl triethyl ammonium chloride) used at different concentration along with an improvement in K/S values after subsequent dyeing with the used metal complex dye. For a given set of finishing and post dyeing conditions, it is clear that: i) increasing the cationic agent concentration results in an improvement in the nitrogen content of the modified fabrics as well as increasing of K/S values after subsequent dyeing, irrespective of the added cationising agent, ii) the extent of improvement is governed by the nature of cationising agent and follows the descending order: Chitosan > Quat-188 > None, reflecting the differences among these agents in molecular weight, chemical composition and functionality, which is a direct consequence of improving the extent of swell-ability of the linen fabrics structure and facilitating the diffusion of the reactants within this structure thereby enhancing the extent of modification of the cellulose structure as well as subsequent dyeing., and iii) the extent of variation in the aforementioned properties is governed by the reactivity and basicity capability of reacting between the nitorgenous additives with the Arkofix NZF and fixation onto the cellulose structure compatibility with other ingredients [16]. It may be concluded, from the obtained results, that the most suitable conc. of each chitosan and also Quat188 is from 5%, which gave the higher values of %N and K/S.
|
4.2. Effect of pH of Cationisation Treatment Bath
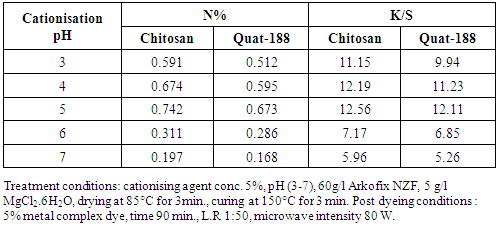
- The pH of cationization treatment bath is an important factor for the reaction of the cationising agents with the linen fabrics. From table 2 it is clear that the appropriate pH for both cationising agents is 5. Increasing the pH led to a decrease in the N% of the treated linen fabrics and the K/S of the dyed linen fabrics. This may be attributed to the absence of H+ ion, which facilitates the protonation of the NH2 of chitosan, thereby reducing the extent of the linen cellulose modification, as well as the extent of the subsequence dyeing [17-19].
|
4.3. Effect of Curing Temperature
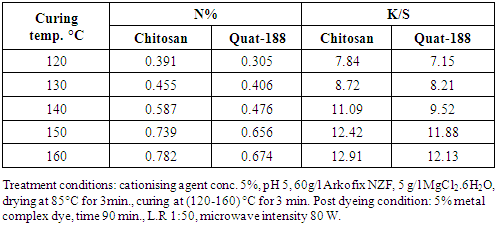
- It was found that from table 3 with increasing curing temperature there was gradually increasing of N% and K/S values of linen as a direct consequence of increasing of modification of the linen fabrics structure regardless of the using cationising agents added. The extent of post dyeing is enhanced also by raising the curing temperature up to 150°C most probably due to fixation of the new active sites on the cellulose structure which cause better dye reaction. Curing temperature 150°C was the optimum degree, noticed that with increasing temperature the fabrics become yellower and cause marginal effect on the aforementioned properties of both additives.
|
4.4. Effect of Curing Time
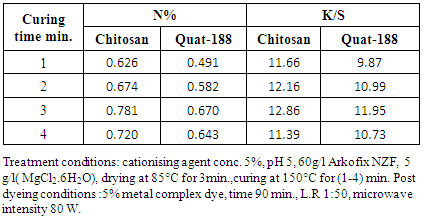
- As far as the changing in N% values of the modified fabrics and K/S values of the post dyed of modified fabrics as a function of curing time, it is clear that from table 4: i) prolonging curing time up to 3 min. Increases N% as well as enhancing the extent of post dyeing i.e. K/S values regardless of the using cationising agents added, that because of the increasing extent of reactions and modification interaction. The curing for 4 min. has practically slight or no effect on the aforementioned parameters.
|
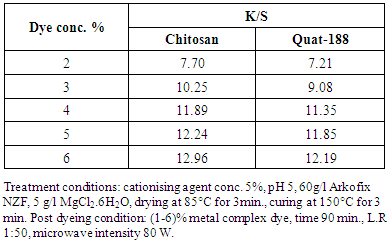
4.5. Effect of Dye Concentration on K/S of the Dyed Linen Fabrics
- The Effect of dye concentration on K/S of the cationized linen fabrics is shown in table 5. It is found that increasing the dye concentration is till 5%, accompanied by increase in the K/S values of the cationized linen samples.Regardless of the cationising agent used. The cationized linen fabrics were found to have high adsorption capacity with the applied metal complex dye. This observation may be attributed: i) electrostatic attraction between positive charges cationic sites, (NH3+) of the cationized linen fabrics and negative charge as found in 1-2 metal complex dye which leads the formation of ionic bonds, “salt linkage" between them, and also formation of some physical forces of attraction between dye molecules and fibers i.e., hydrogen bonding and Van der Waal's forces, ii) the hydrophilic character of linen and facilitates the rapid approach of cationic agent, and iii) the adsorption and formation of cationization film on the fiber surface.
|
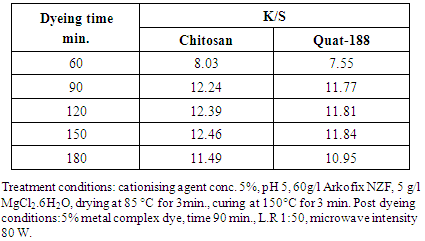
4.6. Effect of Dyeing Time on K/S of the Dyed Cationised Linen Fabrics
- Table 6 shows the effect of dyeing time on K/S of the dyed cationised linen fabrics The dyeing time and its impact on the obtained color strength was studied to determine the suitable dyeing time for achieving maximum color strength.
|
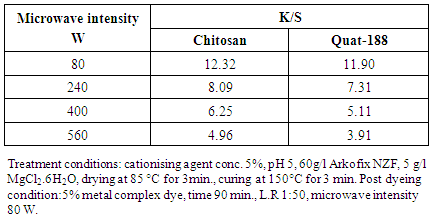
4.7. Effect of Microwave Intensity on K/S of the Post Dyed Cationized Linen
- Table 7 shows the effect of microwave intensity on K/S of the post dyed cationized Linen samples. It is clear that the maximum K/S of the dyed samples was obtain at 80W intensity but above this intensity the K/S decreased. Because of the high temperature, fiber swell-ability is enhanced and, there is an increase in the rate of the dye penetration into the cationised substrate [20-22].
|
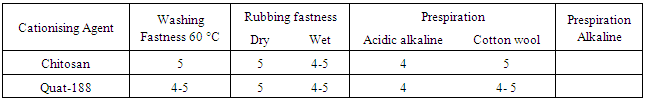
4.8. Fastness Properties
- Table 8 shows the fastness properties of the post dyed cationised linen fabrics. As far as the changing in the fastness properties as a function of the nature of the cationising agent. The washing, rubbing and perspiration fastness properties of the dyed samples are rated quite high because of the higher degree of fixation. Wet rubbing fastness was found to be slightly lower than dry rubbing fastness, most probably due to presence of unfixed dye.
|
5. Conclusions
- Linen fabrics were cationised by using chitosan and Quat-188 in the same conditions which applied using pad -dry -cure technique to give the fabrics a cationic charge. The fabrics were padded in solution contains: cationising agent conc. 5%, pH 5, 60g/l Arkofix NZF, 5 g/l MgCl2.6H2O, then drying at 85°C for 3min., then curing at 150°C for 3 min. After that the fabrics were successfully dyed in the microwave technique in the following conditions 5% metal complex dye, time 90 min., L.R 1:50, microwave intensity 80 W.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML