Mohammad Amir Hamzah1, Ashraful Islam2, Md. Ziaur Rahman3, Md. Hasan Ikbal1, Md. Eman Talukder2, Kamrul Hasan4
1Department of Textile Engineering, Primeasia University, Dhaka, Bangladesh
2Department of Textile Engineering, Southeast University, Dhaka, Bangladesh
3Ministry of Textile & Jute, Textile Vocational Institute, Foridpur, Bangladesh
4Department of Textile Engineering, City University, Dhaka, Bangladesh
Correspondence to: Md. Hasan Ikbal, Department of Textile Engineering, Primeasia University, Dhaka, Bangladesh.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
This research paper contains the assessment of the microbial quality of some medical textiles and some export quality fabrics. For the assessment of microbial quality, different samples of the medical textiles and finished fabrics with antimicrobial finish have been collected from different hospitals located in Dhaka city and from different export oriented textile industries in Bangladesh respectively. Microbial quality of the samples have been determined by total viable bacterial count (TVBC), total coliform count (TCC), total fecal coliform count (TFCC), total staphylococcus count (TSC), total Shigella-salmonellae and total fungi count (TFC). The bacterial isolates from different medical textiles and export quality fabrics have been identified on the basis of microscopic appearance of the microorganism. In microbiological analysis of medical textile, four types of bacteria have been found- aerobic heterotrophic, coliform, fecal coliform, staphylococcus aureus and fungi. After testing the antimicrobial finished fabric for potency, it has been found that the fabrics can prevent the growth of bacteria like aerobic heterotrophic, coliform, fecal coliform, staphylococcus aureus and fungi but their inhibition growth rate is not satisfactory.
Keywords:
Medical textile, Antimicrobial finished textile, Microbial quality, Microorganism, Antimicrobial potency
Cite this paper: Mohammad Amir Hamzah, Ashraful Islam, Md. Ziaur Rahman, Md. Hasan Ikbal, Md. Eman Talukder, Kamrul Hasan, A Comprehensive Analysis on the Efficacy of Antimicrobial Textiles, International Journal of Textile Science, Vol. 4 No. 6, 2015, pp. 137-145. doi: 10.5923/j.textile.20150406.02.
1. Introduction
Bangladesh earns nearly about 80% foreign currency from the textile sectors, exporting casual wears as well as functional clothing. Bangladesh exports the textile products mainly to European region. But, now-a-days this field has become very competitive and facing so much challenges in its business in the competitive global arena. Since most of the raw materials are being imported from abroad and therefore Bangladesh textile sector have been facing two important issues of specifically price and quality of the end products. It is noticeable that the European Buyers are very much conscious about the products they import, particularly the health and hygiene aspects of the end products. Under such situation the producers of textile products in Bangladesh have to be very conscious about the health and hygiene property i.e. antimicrobial property of exportable fabrics. The exportable textiles for medical sectors must be treated with antimicrobial finish and they must have the antimicrobial capability against the activity of the microbes. The term ‘antimicrobial’ refers to a broad range of technologies that can provide varying degrees of protection for textile products against microorganisms. Antimicrobials are defined as the agents that either kill microorganisms or simply inhibit their growth. Antimicrobial finish is a recent innovation in textile finishes. The consumers are now increasingly aware of the hygiene life style and there is a necessity and expectation for a wide range of textile products finished with antimicrobial properties. This kind of finishes prevents the growth of bacteria and unpleasant odor [1].The degree of activity of antimicrobial agent is denoted by, Cidal (agent that kill microorganisms and static agents that inhibit microorganisms’ growth). Antimicrobial textiles can act in two distinct ways of by contact and by diffusion. Disease causing bacteria comes to human health via microorganisms, while clothes are in close contact with the skin, long periods between washings and a special microclimate can favor a fast growth of germs. They come to the human health, penetrate inside the skin and damage the host tissues. Fungi, algae, and viruses which can not be seen by naked eyes can cause serious diseases to human health. The growth of microbes aggravates particularly in humid and warm environment. The result of microbial attack is the staining and loss of the performance properties of textile substrates [2-3].Antimicrobial finish is used on textile materials to protect the wearer and the textile substrates by controlling bacteria, fungi, mold, mildew and algae. Antimicrobial treatment for textile materials is necessary to avoid cross-infection by pathogenic microorganisms, to control the infestation by microbes, to save the textile materials from staining, discoloration and deterioration, to control the formation of odors, allergies and other health concerns that they cause. Microorganisms cause problems with textile raw materials and processing chemicals, wet processes in the mills, roll or bulk goods in storage, finished goods in storage and transport and goods as they are used by the consumers. This can be extremely critical to operator a clean room, a medical facility, or a food processing facility or it can be an annoyance and aesthetic problem to the athlete or normal consumers. Antimicrobial agents kill microorganisms or inhibit their growth by Cell wall damage, Inhibition of cell wall synthesis, Alteration of cell wall permeability, Inhibition of synthesis of proteins and nucleic acids, Inhibition of enzyme action. A microorganism is an organism that is microscopic. Most microorganisms are single-celled or unicellular. Microorganisms are responsible for the degradation of cotton. They never attack the substrate directly where they live. They produce very complex non-living molecules called enzyme which can degrade the cellulose structure. Enzyme forms complicated chemical reactions under mild conditions. The steps of degradation may occur including the separation of different structural units and then hydrolytic break down of single chains at glycoside rings and then finally converts into carbon dioxide (CO2) and water while enzymes degrade cellulose chains [2, 4, 6].
1.1. Textile Products Used in Medical Sector
Any textile structure which has been designed and produced for the uses of medical applications, including implantable applications is to be called medical textile. The engineering approach to develop textile products that will be suitable for medical and surgical application should possess a combination of the properties including strength, flexibility, antimicrobial and moisture and air permeability. The current uses of textiles in Bangladesh may broadly be classified into two groups. One is Non-plantable materials and the other is health and hygiene products.Non-plantable materials are used for external application on the body and may or may not make contact with skin. Some examples of this kind of products are Wound care products, Bandages, Gauze, Lint/Cotton.Solving the problem of healthcare-associated infections and occupationally acquired infections involves an equation with many complex variables. One of the key components is healthcare workers (HCWs), such as doctors, nurses, laboratory personnel and technical professionals, who are frequently exposed to blood and body fluids. These fluids can transmit bacteria that cause colonization or infection, including multi-drug-resistant organisms (MDROs) such as meticillinresistant Staphylococcus aureus (MRSA), Acinetobacter spp. and Enterobacteriaceae (e.g. Escherichia coli, Klebsiella pneumoniae). There is also a risk of transmission of viruses, including noroviruses, respiratory viruses and bloodborne viruses (human immunodeficiency virus, hepatitis B and C viruses), that can survive for hours or days on surfaces [8-10].Healthcare and hygiene products are important sectors in the field of medicine and surgery. The range of products available is vast but typically they are used either in the operation theatre or on the hospital ward for the hygiene, care and safety of staff and patients. Textile materials used in the operating theatre include surgeon’s gowns, caps and masks, patient drapes and cover cloths of various sizes. It is essential that the environment of the operating theatre is clean and a strict control of infection is maintained.
1.2. Factors Affecting the Growth of Microorganism in Fabric
Storage temperature, pH, Relative Humidity of environment, Moisture content, Laundering are the most important controlling factors in case of fabric storage to avoid the growth of microorganisms. Microorganisms grow over a very wide range of temperatures (250c – 300c). The quantity of the textile product must be taken into account in selecting a storage temperature at refrigerator temperature or below. The relative Humidity (RH) of the storage environment is also an important parameter to maintain. Fabric that undergoes surface spoilage from molds, yeast and certain bacteria should be stored under conditions of low RH. It is now generally accepted that the water requirements of microorganisms should be defined in terms of water activity. Laundering also causes little observable surface damage to cotton fiber but it is observed that damage to cotton fiber by microbial attack increases with each laundering. Severe damage is noticed after 10th laundering, suggesting that possible physical breakdown may make the fibers more accessible to microbial attack [3, 5].
1.3. Modern Applications on Cotton Fabrics for Antimicrobial Property
As the awareness of healthcare increasing and the global infectious disease events frequently happening, a wide range of researches has been done to obtain antibacterial textiles which could not only avoid discoloration and degradation of fabrics by microorganisms but also effectively prevent the propagation of bacteria. The inherent chemical and morphological properties of cellulose fibers are reflected in their biodegradability and high hydrophilicity, providing an excellent medium for bacterial growth. Therefore, cellulose products with antimicrobial properties can only be obtained by appropriate chemical modification of the fibers. Cellulose fibers with antimicrobial properties are an essential textile material that can be used in various types of products with applications in apparel, technical, medical and hygienic purposes as well as in the food industry [11].
2. Materials and Methods
2.1. Source of Fabric Collection
Medical Textile: Medical textile samples have been collected from different hospitals, situated in Dhaka city (Samples have been collected by ensuring the authority that their name would not be used anywhere in the paper.)Export Oriented Fabrics: Reedisha Knit Composite and Square Knit, 36, Shahid Tajuddin Ahmed Sarani, Tejgaon Industrial Area, Dhaka-1208, Bangladesh.
2.2. Description of the Samples
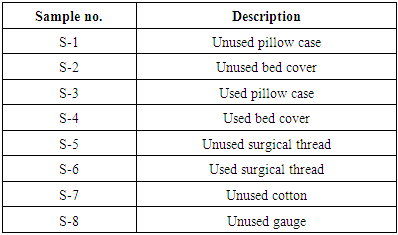
Eight samples of medical textiles (Table 1) and three samples of antimicrobial finished exportable fabrics (Table 2) have been examined for this study.Table 1. Description of medical textiles samples
 |
| |
|
Table 2. Description of the export oriented fabric samples
 |
| |
|
2.3. Sample Preparation Procedure
The collected samples inserted in to the conical flask, containing 250 ml of sterilized water. In this way, the required number of samples were taken and transferred to the microbiological laboratory for investigation.
2.4. Test Techniques
Two test techniques have been followed:● Microbiological analysis of medical textiles.● Antimicrobial potency of finishing agents used on exportable textile cloths.
2.4.1. Test Procedure for Microbial Analysis of Medical Textiles
Sample preparation:One gram of the sample was weighed aseptically into a sterile test tube and 9 ml of sterile normal saline was added into it. One gram of homogenate was transferred to a test tube containing 9 ml sterile normal saline to make 10-2 dilution and was shaken well with vortex mixture. A sterile dilution up to 10-3 was also made in the same process.Sterilization:All equipment and glassware were sterilized by autoclaving at 121°C temperature and 15 p.s.i. for 15 minutes in the microbiology laboratory. All growth media and solutions were sterilized by the same process. Fresh agar plates were prepared at room temperature, stored at 4°C until use and dried at room temperature under aseptic conditions before inoculation of samples.Growth media:Dried media such as nutrient agar, mFC agar, Mac-Conkey agar, potato dextrose agar, mannitol salt agar (MSA) and Shigella-Salmonellae agar (SS-agar) were prepared in the microbiology laboratory.Maintenance of culture, reagents and solutions:Preservation and maintenance of different materials including microbial stains, heat labile chemicals and reagents etc. were carried out in a refrigerator at 4°C temperature. Nutrient agar slants were used for the routine maintenance of the pure bacterial strains. The pure bacterial cultures were stored at 4°C until use.All culture was inoculated under aseptic conditions. The organisms were inoculated in a laminar airflow cabinet. The inoculated solid and liquid media for static growth conditions were incubated using binder indicator. In incubator Petridis is kept overnight at 37°C. Then a colony counter was used to count the microbial colonies on solid agar plates.
2.4.2. Test procedure for Antimicrobial Finishing Agents Used on Exportable Textile Cloths
The fabric sample was cut into small pieces (1 sq. Inch) in aseptic condition and kept them in the sterilized conical flask containing 100 ml of sterilized water. The conical flask was kept at room temperature for 48 hours for the diffusion of antimicrobial agent to the water. The known microbial samples were prepared with normal saline and diluted for the microbial counts. The same dilution was also used for the challenge test. Diffused solution from fabrics was then used as samples for the potency test. 100 ml of microbial sample (known microbial counts) of diffused was then separated on to the agar medium and 100 ml of diffused fabric solution as a challenge was added to find out the antimicrobial potency which had been added in the supplied fabric samples.
2.5. Examining the Presence and Measure of Microorganisms or Bacteria in Different Textile Products
The bacterial count was done by standard method of ICMSF. The microbiological condition of safety and hygiene were examined by using the methods recommended by ICMSF.
2.5.1. Total Viable Bacterial Count (TVBC)
Total Viable Bacterial Count test was carried out to find the total microbial counts and for the quality assessment of the products [5].Total viable bacteria count was carried out by the spread plate technique. Petri plates were sterilized in hot air oven at 180°C for two hours. All types of media used after sterilization, autoclaved at 121°C with 15 p.s.i. for 15 minutes. The homogenate sample (0.1 ml) of each dilution was taken onto each Petri dish. Then the sample was finely spread on the solid nutrient medium. The prepared dishes were then incubated at 37°C for 24 hours for counting mesophilic bacteria. The plates were then screened (using a colony counter) for the presence of discrete colonies after the specific incubation period and the actual numbers of bacteria was estimated as cfu/gm and finally the results per dilution will be recorded. All colonies on dishes containing 30-300 colonies were counted using a colony counter. The actual number of bacteria was estimated as cfu/gm and the results per dilution counted were recorded. Figure 1 shows the viable bacteria containing plate from which total viable bacteria was counted. Viable bacteria has been detected on plate count agar in all the 8 samples of hospital materials.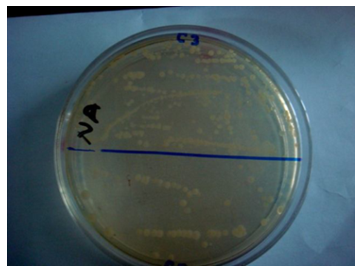 | Figure 1. Assessment of Total Viable Bacteria Count (TVBC) on different Medical Textiles |
2.5.2. Total Coliform Count (TCC)
Total Coliform Count (TCC) test was carried out to find the presence of pathogenic bacteria on textile materials, which are responsible for food poisoning, diarrhea, etc. [5].Mac-Conkey agar media was used for counting of total coliform bacteria. Homogenate sample (0.1 ml) from each dilution was taken onto each petri plates. Then the sample was finely spread onto the solid medium and the prepared dishes were incubated at 37°C for 24 hours. Then the plates were screened (using a colony counter) for the presence of discrete colonies after the specific incubation period and the actual numbers of coliform was estimated as cfu/gm. Then the results per dilution were recorded. Figure 2 shows the detection of total coliform containing agar plate from which total coliform was counted. The total coliform count was detected by using Mac-Conkey agar media and it has been found in all 8 tested samples of medical materials.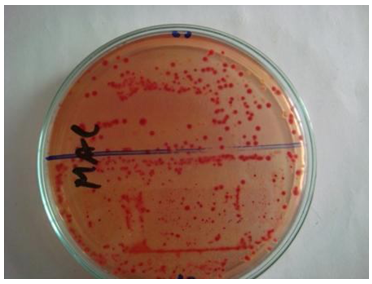 | Figure 2. Assessment of Total Coliform Count (TCC) on different Medical Textiles |
2.5.3. Total Fecal Coliform Count (TFCC)
Total Fecal Coliform Count (TFCC) test has been done to find out the presence of pathogenic bacteria, which are responsible for diarrhea, bloody dysentery etc. [5].mFC agar medium was used for counting total fecal coliform. Homogenate sample (0.1 ml) from each dilution was taken onto each sterile Petri dish. Then the sample was homogeneously distributed on the plate using a glass spreader in a backward and forward movement while rotating the plate and the plates were incubated at 45°C for 24 hours. Then the plates were screened for the presence of discrete colonies after the specific incubation period and the actual numbers of Fecal coliform were estimated as cfu/gm. Then the results per dilution were recorded. Figure 3 shows the fecal coliform containing plate from which fecal coliform was counted. No fecal coliform has been found in the samples of medical textile materials.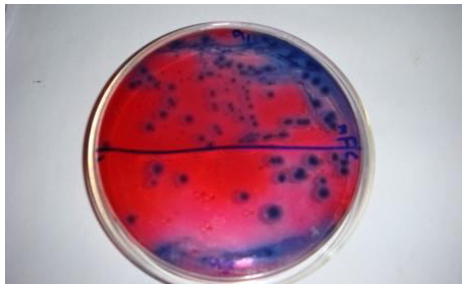 | Figure 3. Assessment of Total Fecal Coliform Count (TFCC) on different Medical Textiles |
2.5.4. Total Shigella-Salmonellae Count (TSSC)
Total Shigella-Salmonellae Count (TSSC) test has been performed to find out the presence of deadly pathogenic bacteria. The Salmonellae may be transmitted by water, air, food or more really direct anal-oral transmission. Typhoid fever is caused by Salmonellae typhi [5].Figure 4 shows the Shigella-Salmonellae containing plate from which Shigella-Salmonellae was counted. No Shigella-Salmonellae has been found from the samples of medical textiles.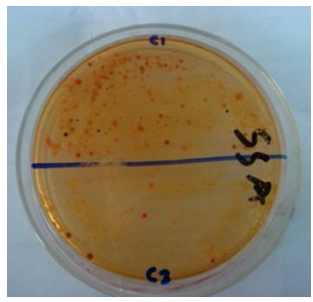 | Figure 4. Assessment of Total Shigella-Salmonellae Count (TSSC) on different Medical Textiles |
2.5.5. Staphylococcus Aureus Count
Staphylococcus Aureus Count testing was carried out to observe the presence of puss forming bacteria. Levels of Staphylococcus Aureus greater than or equal to 104 cfu/gm are considered as potentially hazardous for skin [5].Mannitol salt agar medium was used for this purpose. Homogenate sample (0.1 ml) from each dilution was taken onto each sterile petri dish. Then the sample was homogenously distributed on to the plate using a glass spreader in a backward and forward movement while rotating the plate and then the plates were incubated at 37°C for 24 hours and screened for the presence of discrete colonies after the specific incubation period. Figure 5 shows the staphylococcus containing plate from which Staphylococcus aureus was counted. By using Mannitol salt agar medium staphylococcus aureus count of different hospital materials were detected.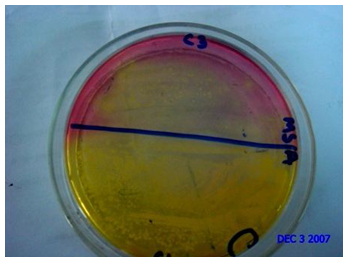 | Figure 5. Assessment of Staphylococcus Aureus Count on different Medical Textiles |
2.5.6. Total Fungi Count (TFC)
Total Fungi Count (TFC) test has been done to observe the presence of yeast and mold that are responsible for skin disease [5]. By using potato dextrose agar medium, fungi count was detected from different hospital materials. Sabouraud dextrose agar medium was used for this purpose. Homogenate sample (0.1 ml) from each dilution was taken onto each sterile petri dish. Then the sample was homogenously distributed on the plates using a glass spreader in a backward and forward movement while rotating the plate. Then the plates were incubated at 25°C for 5 days. Then all the colonies on dishes were counted using a colony counter and the actual number of fungi was estimated as cfu/gm. The results per dilution were recorded.
3. Result and Discussion
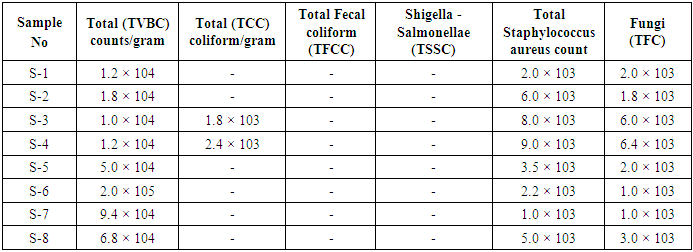
3.1. Microbiological Test Result of Medical Textiles
Table 3. Microbiological analysis of medical textiles
 |
| |
|
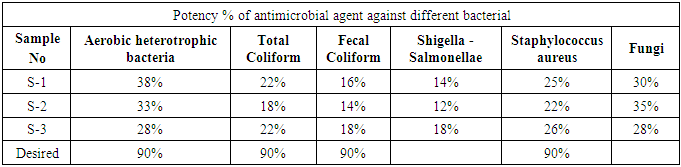
3.2. Potency of Antimicrobial Agents Used on Fabrics
Table 4. Potency of Antimicrobial Agents used on Fabrics
 |
| |
|
3.3. Microbial Result Analysis of Medical Textiles
3.3.1. Total Viable Bacteria Count
From Figure 6 it can be seen that, the minimum count was 1.0 × 104 and the highest count was 2.0 × 105. The standard tolerance for Total Viable Bacteria Count (TVBC) should be 5.0 × 104.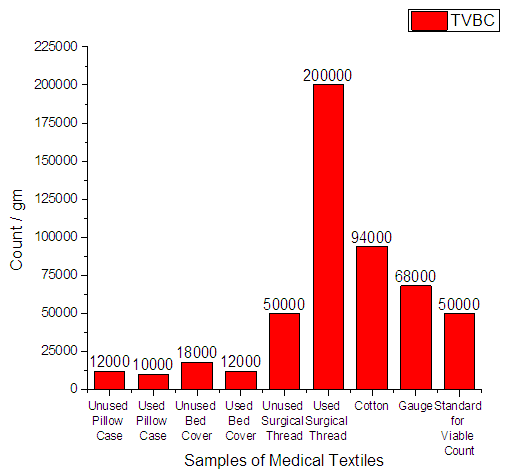 | Figure 6. Total Viable Bacteria Count on Medical Textile |
3.3.2. Total Coliform Count
From Figure 7 it can be seen that, total coliform were found in 2 samples only-used pillow case and used bed cover. The standard value for Total Coliform Count (TCC) should be only 3.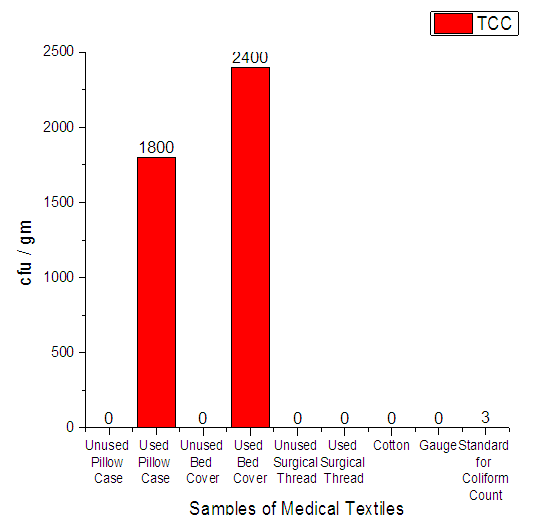 | Figure 7. Assessment of Total Coliform count on Medical Textile |
3.3.3. Staphylococcus Aureus Count
From Figure 8 it can be seen that, the total number of staphylococcus aureus in 8 samples of hospital materials was detected on plate count agar. Staphylococcus aureus were found in all the samples. The minimum count was 2.0 × 103 and the highest count was 3.5 × 104. The standard tolerance for Staphylococcus aureus should be 0.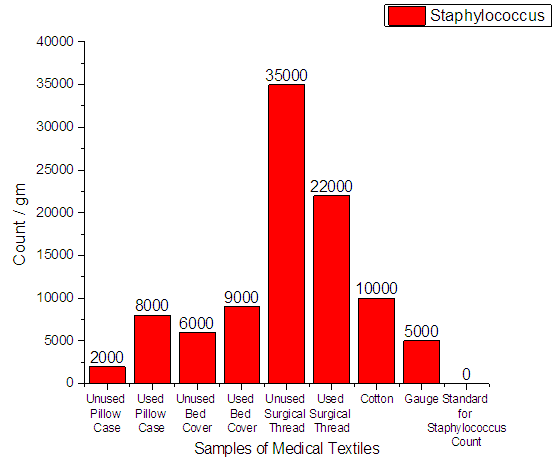 | Figure 8. Assessment of Staphylococcus Aureus Count of medical textile |
3.3.4. Fungi Count
From Figure 9 it can be seen that, the total number of fungi in 8 samples of hospital textile materials was detected on count plate agar. Fungi were found in all the samples. The minimum count was 1.1 × 103 and the highest count was 6.4 × 103. The standard value for fungi count should be 0.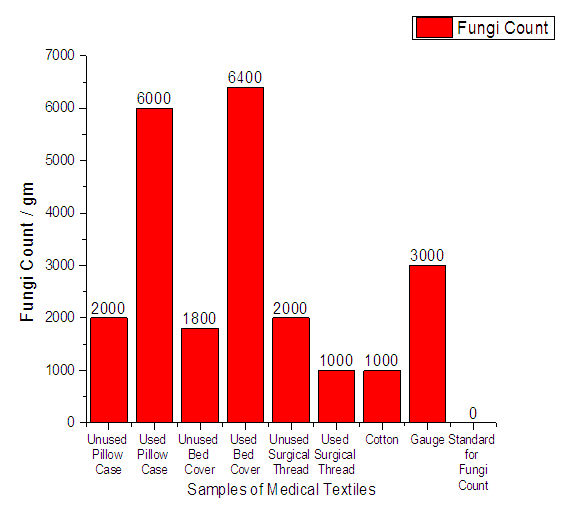 | Figure 9. Assessment of Staphylococcus Fungi Count of medical textile |
3.4. Result Analysis (Potency of Antimicrobial Agents Used in Fabrics)
3.4.1. Aerobic Heterotrophic Bacteria
From Figure 10 it can be seen that, sample 1, 2, & 3 can prevent 38%, 33% and 28% Aerobic heterotrophic bacteria respectively. The more the sample can inhibit the growth of bacteria means antimicrobial finish is successful. The desired rate of inhibition the growth of bacteria should be 90%.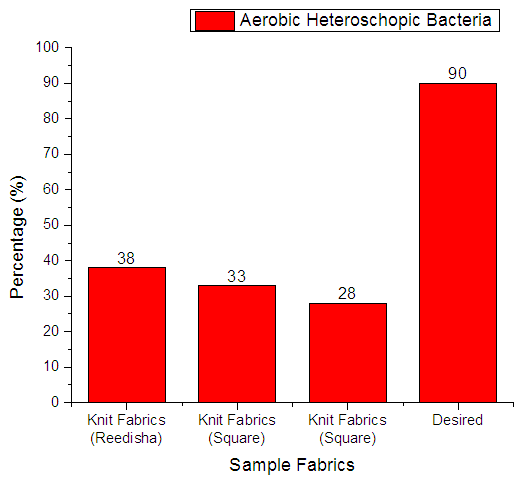 | Figure 10. Assessment of antimicrobial agent used on export quality fabrics by Aerobic heterotrophic bacteria |
3.4.2. Total Coliform
From Figure 11 it can be seen that, sample 1, 2, & 3 can prevent 22%, 18% and 22% total coliform respectively. The more the sample can inhibit the growth of bacteria means antimicrobial finish is successful. The desired rate of inhibition the growth of bacteria should be 90%.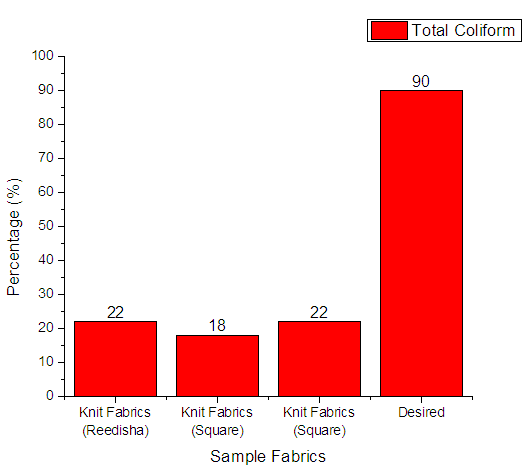 | Figure 11. Assessment of antimicrobial agent used on export quality fabrics by Aerobic heterotrophic bacteria |
3.4.3. Fecal Coliform
From Figure 12 it can be seen that, sample 1, 2, & 3 can prevent 16%, 14% and 18% fecal coliform respectively. The more the sample can inhibit the growth of bacteria means antimicrobial finish is successful. The desired rate of inhibition the growth of bacteria should be 90%.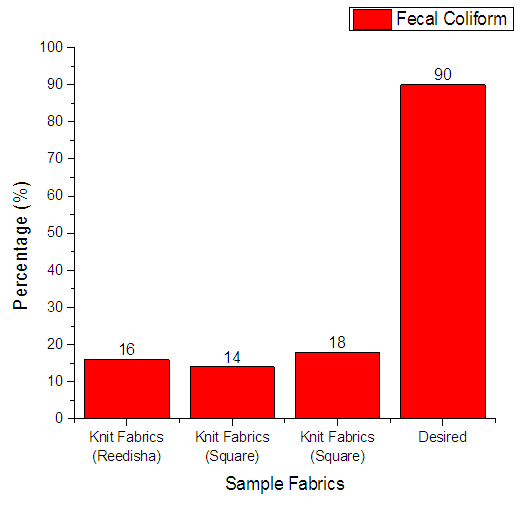 | Figure 12. Assessment of antimicrobial agent used on export quality fabrics by Fecal Coliform bacteria |
3.4.4. Shigella-Salmonellae
From Figure 13 it can be seen that, sample 1, 2, & 3 can prevent 14%, 12% and 18% Shigella-Salmonellae respectively. The more the sample can inhibit the growth of bacteria means antimicrobial finish is successful. The desired rate of inhibition the growth of bacteria should be 90%.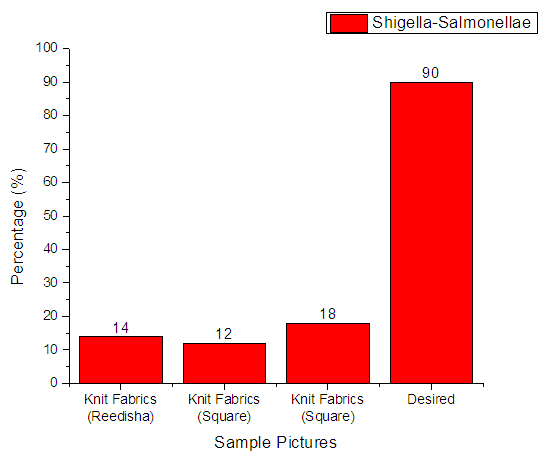 | Figure 13. Assessment of antimicrobial agent used on export quality fabrics by Shigella-Salmonellae bacteria |
3.4.5. Staphylococcus Aureus
From Figure 14 it can be seen that, sample 1, 2, & 3 can prevent 25%, 22% and 26% Staphylococcus Aureus respectively. The more the sample can inhibit the growth of bacteria means antimicrobial finish is successful. The desired rate of inhibition the growth of bacteria should be 90%.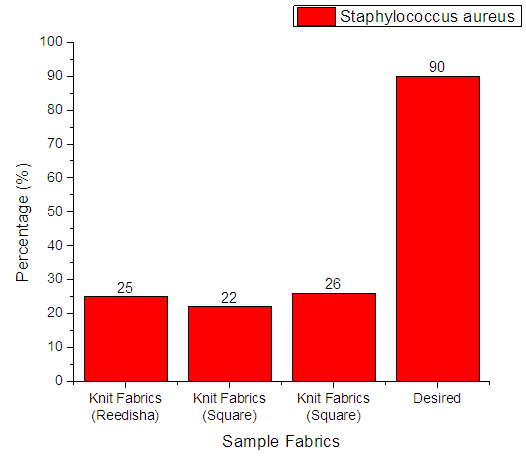 | Figure 14. Assessment of antimicrobial agent used on export quality fabrics by Staphylococcus Aureus bacteria |
3.4.6. Fungi Count
From Figure 15 it can be seen that, sample 1, 2, & 3 can prevent 30%, 35% and 28% Fungi respectively. The more the sample can inhibit the growth of bacteria means antimicrobial finish is successful. The desired rate of inhibition the growth of bacteria should be 90%.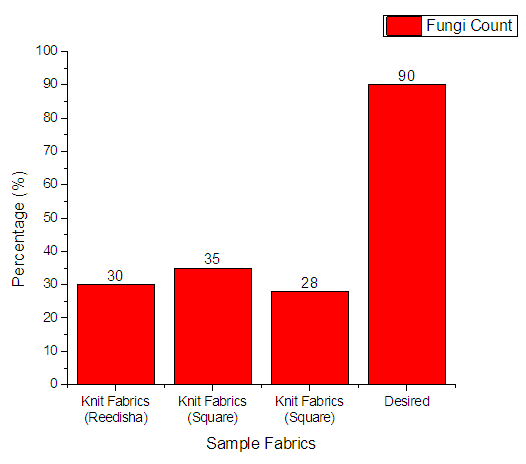 | Figure 15. Assessment of antimicrobial agent used on export quality fabrics by Fungi Count |
4. Conclusions
This paper mainly divided in to two major part of microbial quality assessment of the textiles used in medical sectors or hospitals and antimicrobial exportable finished fabrics. The tests performed to investigate those criteria include: Total Viable Bacteria Count (TVBC), Total Coliform Count (TCC), Total Fecal Coliform Count (TFCC), Total Staphylococcus Count (TSC), Total fungi Count (TFC).In the result of microbiological assessment of medical textile, TFCC and Shigella-Salmonellae were found to be entirely absent. Therefore, it may be concluded that the textiles used in medical sectors were good since they do not contain the most pathogenic bacteria Shigella-Salmonellae. A point is to be noted again that these types of bacteria are responsible for typhoid, dysentery, water and food borne diseases. The standard tolerance for Staphylococcus aureus should be 1.0 × 102, but in the test result it has been found that the minimum value of it is 1.0 × 104 and the maximum value is 9.0 × 103. The values found cross the standard limit. This type of bacteria is particularly responsible skin disease. The TCC was also found in the test results that cross the standard limit which is 3.0 cfu/gm only. The minimum value for TCC found is 1.8 × 103 and the maximum value of it found to be 2.4 × 103. The TCC bacteria are responsible for urinary tract infections, respiratory tract infections and other extra intestinal infections. Fungi were also found in the test result that cross the standard limits 0 (zero). The minimum value of it found to be 1.0 × 103 and maximum value 6.4 × 103. The fungi are large organisms and responsible for cancer and malnutrition. The research has found that the textiles used in medical or hospitals do not contain the most pathogenic bacteria Shigella-Salmonellae and total fecal coliform which are regarded to be very harmful. On the other hand the textile materials contain fungi types of microbes which are very harmful.To identify the potency of antimicrobial finishing agent six types of tests have been carried out. The standard level of potency of antimicrobial agent should be 90% which although can be attained. In this work, the minimum efficacy for Aerobic heterotrophic bacteria has been found to be 28% and maximum 38%. The maximum efficacy for coliform has been found to 18% and maximum 28%. The maximum efficacy for fecal coliform has been found to be 14% and maximum 18%. The maximum and minimum values for staphylococcus have been found to be 22% and 26% respectively. The maximum and minimum values for Shigella-Salmonellae have been found to be 12% and 18% and for Fungi the values are 28% and 35% respectively. With reference to the results it may be concluded that collected antimicrobial finished fabric are able to inhibit the growth of microorganisms and their inhibition growth rate is not satisfactory.
ACKNOWLEDGEMENTS
This research work has been done in the microbiology laboratory of Primeasia University.
References
| [1] | H. Mucha, D. Hoter and M. Swerev ‘Antimicrobial Finishes and Modifications’ Melliand International, May 2002, vol. 8, p-148-151 and I. Home. ‘Antimicrobials Impart Durable Finishes’ International Dyer, December 2002, p-9-11. |
| [2] | New Multifunctional Textiles: Antimicrobial Treatments, ‘Intelligent textile structures-application, production and testing’ 12-13/5/2005, Thessaloniki, Greece, amphitheater of Thessaloniki Technology Park. |
| [3] | ‘Antimicrobial Textiles: an Overview’ Dr. T. Ramachandran, Member, K Rajendrakumar, Non-Member, and R. Rajendran, Non-Member. |
| [4] | H. Mucha, D. Hoter and M. Swerev, ‘Antimicrobial Finishes and Modifications’ Melliand International, May 2002, vol. 8, p-148-151, I Home ‘Antimicrobials Impart Durable Finishes’, International Dyer, December 2002, p-9-11 and D Gupta ‘Antimicrobial Finishing of Textiles’ www.resil.com. |
| [5] | Bauchop T. & Elsden S. R. ‘The growth of microorganisms in relation to their energy supply’ Journal of General Microbiology, 1960 and Tempest D. W. ‘The biochemical significance of microbial growth yields: a reassessment TIBS’ Elsevier/North Holland Biomedical Press, 1978, p-180. |
| [6] | Linko, M. ‘An evaluation of enzymatic hydrolysis of cellulosic materials’, in Advances in Biochemical Engineering, 5, 39, 1977, Bertran, M.S. and Dale, B.E. ‘Enzymatic hydrolysis and recrystallization behavior of initially amorphous cellulose’, Biotech. Bioeng, 27, 177, 1985 and Ghose, T.K. ‘Cellulase biosynthesis and hydrolysis of cellulosic substances’, in Advances in Biochemical Engineering, 6, 25, 1977. |
| [7] | ‘Statistical Pocket Book of Bangladesh (1st Edition, 2007). Bangladesh Bureau of Statistics. |
| [8] | Mitchell A, Spencer M, Edmiston C, Role of healthcare apparel and other healthcare textiles in the transmission of pathogens: A review of the literature, Journal of Hospital Infection (2015), doi: 10.1016/j.jhin.2015.02.017. |
| [9] | Hawkins G, Stewart S, Blatchford O. Should HCWs be screened routinely for methicillinresistant Staphylococcus aureus? A review of the evidence. J Hosp Infect 2011;77:285–289. |
| [10] | Ibarra M, Flatt T, Van Maele D, Ahmed A, Fergie J, Purcell K. Prevalence of methicillinresistant Staphylococcus aureus nasal carriage in HCWs. Pediatr Infect Dis J 2008;27: 1109-1111. |
| [11] | Ferrero, F., et al., Sustainable antimicrobial finishing of cotton fabrics by chitosan UV-grafting: from laboratory experiments to semi industrial scale-up, Journal of Cleaner Production (2013),http://dx.doi.org/10.1016/j.jclepro.2013.12.044. |

















 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML


