-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Textile Science
p-ISSN: 2325-0119 e-ISSN: 2325-0100
2015; 4(5): 102-112
doi:10.5923/j.textile.20150405.02

New Azo Disperse Dyes with Thiophene Moiety for Dyeing Polyester Fibers
1Chemistry Department, Faculty of Science, Al-Azhar University, Nasr City, Cairo, Egypt
2Department of Chemistry, Faculty of Arts and Science, Albaha University, Mukhwah, KSA
Correspondence to: H. S. Nassar, Chemistry Department, Faculty of Science, Al-Azhar University, Nasr City, Cairo, Egypt.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The synthesis of two series of some new bis-hetaryl monoazo dyes from diazotized phenatidinyl-5-amino- thiophene derivatives and 4-aryl-2-aminothiazoles and 4-aryl-3-substituted-2-aminothiophens derivatives as coupling components, respectively, was described. Structural characterization of these novel dyes was carried out. The visible absorption maxima of dyes shift large bathochromic was preformed, in basic organic solvent with high polarity (dimethylformamide) relative to corresponding solvent with low polarity (ethanol). These dyes were applied as disperse dyes for dyeing polyester fibres. The dyed fabric show good light fastness, very good rubbing, perspiration, washing and excellent sublimation fastness. These dyes have been color shade from grey to blue with very good depth and levelness on fabrics, and fastness properties.
Keywords: Aminothiophenes, Azo disperse dyes, Polyester fibers, Fastness properties
Cite this paper: H. S. Nassar, New Azo Disperse Dyes with Thiophene Moiety for Dyeing Polyester Fibers, International Journal of Textile Science, Vol. 4 No. 5, 2015, pp. 102-112. doi: 10.5923/j.textile.20150405.02.
Article Outline
1. Introduction
- Interest in the design of azo dyes containing heterocyclic moieties stems from their high degree of brightness compared to azo dyes derived from anilines [1-11]. The 2-aminothiophene based azo dyes are known as disperse dyes with excellent brightness of shade. This class of dyes was established as an alternative to more expensive anthraquinone dyes [12, 13]. The thiophene-containing azo dyes have many advantages including a colour deepening effect as an intrinsic property of the tiophene ring and small molecular structure leading to better dye ability. The heterocyclic nature of the thiophene ring has also allowed for excellent sublimation fastness on the dyed fibers [14, 15], Increasing the electron-with drwing strength of the substituents on the thiophene ring resulted in bathochromic shifts [16]. Additionally, the sulfur atom plays a decisive role by acting as an efficient electron sink as explained by valence band theory [17].A one-pot procedure by Gewald [18, 19] created an efficient route to the synthesis of thiophenes based heterocyclic dyes. A number of researchers studied aminothiophene derivatives as azo disperse dyes in the dyeing of synthetic fibers [20-28], blende polyester/wool fibers [29, 30], and more recently in optical data storage devices [31]. This class of compounds also showed semiconducting properties [32].Other research program has been focused on the incorporation of aryl and hetaryl azo dyes in or pendent to the backbone of polynorbornenes, polyether and polythioethers [33-35]. In light of the interesting cis-trans photoisomerization behavior, these materials may have potential optical applications.In earlier works, we reported the synthesis of some bishetaryl monoazo dyes drived from both heterocycles 2-pyridones and 5-pyrazolones, phenol or naphthol derivatives and/or amine as coupling components [36-38]. The obtained dyes exhibit their colour hue in the range from yellow to violet. Moreover, Azo disperse dyes containing 2-aminothiophenes as coupling component have also been described as having red to violet colors in literature [12, 26]. In the present work, p-acetophenetidide were applied using Gewalds reaction for the synthesis of 2-aminothiophenes. The resulting aminothiophenes were diazotized and coupled with 4-aryl-2-aminothiazoles and 4-aryl-3- substituted -2- aminothiophens, respectively, the obtained dyes show deep grey to blue colour hue.The couplers affording a variety of azo dyes based on thiophene moieties. Using the same strategy, the synthesis of aminothiophenes can be further broadened, by using p-Acetophenetidide with electron-donating or electron - withdrawing groups to yield a new wider variety of aminothiophenes prepared directly by Gewalds synthesis.
2. Experiment
- All melting points were measured on a Stuart Scientific Co. Ltd (UK) melting point apparatus. Infrared spectra were recorded on a Fourier transform infrared spectroscopy (FT-IR) Bruker Vector 22 spectrophotometers using KBr wafer technique. ultraviolet visible spectra (UV-Vis) were recorded with a Perkin-Elmer Lambda-3B. 1H Nuclear magnetic resonance spectra (NMR) were measured with a Varian Mercury 300 (300 MHz 1H NMR, 75 MHz 13C NMR), spectrophotometer and chemical shifts are given in parts per million from transmission monitor system (TMS). Mass spectra were recorded on a gas chromatograph-mass spectrometer spectra (GC-MS) QP1000 EX (70 eV). The substrate used for dyeing (100% polyester fiber) was Woolen type, provided by from Misr Rayon company, Kafr El-Dawar, Egypt. All applications, fastness properties and color measurements of the dyes were studied in Laboratories and Reasearch sector in Misr Company, for Spinning and Waving, Mehalla El-Kobr, using the colour matching computer ACS-600 colour control system. All fastness properties of the dyes were studied in El-Nasr Company for spinning, weaving and dyeing at Mehalla El-Kobra, Egypt. Elemental analyses were carried out at the Microanalytical Centre, Cairo University.
2.1. Synthesis of Starting Materials and Intermediates
2.1.1. Synthesis of p-acetophenetididen 2
- General Procedure:A mixture of amine 1 (0.1 mol) and ethylacetoacetate (0.1 mol) was heated at 150oC with constant stirring. The reaction mixture was allowed to stand at room temperature for 1 h, and pour into petroleum ether 40/60, then the solid was collected and recrystallized from ethanol to give 2. [39]. These compounds are identical m.p. Their analytical data agreed with the published work.
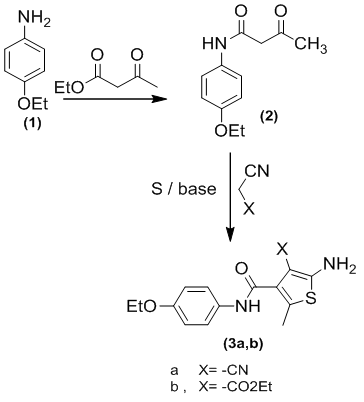
2.1.2. Synthesis of Thiophene Intermediates (3a,b)
- Aminothiophenes derived from 1,3-dicarbonyl compounds, active α-methylene nitriles or ethylcyanoacetate, and sulfur were prepared using a "one-pot" Gewald reaction [40].
2.1.2.1. 5-Amino-4-cyano-2-methylthiophene-3-carboxylic acid p-phenetylamide (3a)
- p-Acetophenetidide 2 (1.79 g,0.1 mol), malononitrile (6.96 g, 0.1 mol), and sulfur (3.37 g, 0.1 mol) in ethanol were stirred in the presence of morpholine (8.97g, 0.1 mol) at 60-70°C for 3 hrs. The resulting thick dark solution was cooled and stored overnight in a refrigerator, followed by filtration. Washing with ethanol, then ethanol/water solution and dried. Yield 73%, mp 292-294°C. IR spectrum of compound exhibited absorption bands max./cm-1 at 3344.2, 3316.4, 3199.0 (NH / NH2), 2979.6 (CH.), 2217.0 (CN), 1620.8 (CO). 1H NMR spectrum of compound (DMSO-d6) showed signals at : 1.25 (t,3H, -OCH2CH3), 2.37 (s,3H, -CH3), 4.15 (q,2H, -OCH2CH3), 6.87-7.35 (dd,4,H, AB-system, ArH's), 7.92 (s,2H,NH2; exchangeable with D2O), 9.40 (s,1H,NH; exchangeable with D2O). MS (70eV) m/z (rel. intensity): 301 (34.2), 137 (100.0), 66 (16.9). Anal. Calc. for C15H15N3O2S (301): C, 59.78; H, 5.02; N, 13.95. Found: C, 5971; H, 4.90; N, 13.90.
2.1.2.2. Ethyl-2-amino-4-((4-ethoxyphenyl)carbamoyl) -5-methylthiophene-3-carboxylate(3b)
- p-acetophenetidide 2 (1.79 g,0.1 mol) was stirred with ethalcyanoacetate (6.96 g, 0.1 mol), and sulfur (3.37 g, 0.1 mol) in ethanol. The morpholine (8.97g, 0.1 mol) was added in the reaction mixture, which continued at 60-70°C for 3 hrs. The resulting thick dark solution was cooled and stored overnight in a refrigerator, followed by filtration. Washing with ethanol, then ethanol/water solution and dried. Yield 74%, mp. 277-279° C. IR spectrum exhibited absorption bands max./cm-1 at 3425., 3107.2, 3297.5 (NH/NH2), 2978.9 (CH), 1668 (CO).). 1H NMR (DMSO-d6) showed signals at : 1.12 (t,3H, -OCH2CH3), 1.27 (t,3H, -OCH2CH3), 2.62 (s,3H, -CH3), 4.10 (q,2H, -OCH2CH3), 4.23 (q,3H, -OCH2CH3) 6.95-8.05 (.dd,4H,AB-system ArH's), 7.77 (2s,2H, NH2) 8.5 (s,1H,NH). MS (70eV) m/z (rel. intensity): 348 (41.47), 137 (100.0), 66 (26.9). Anal. Calc. for C17H20N2O4S(348): C, 58.60; H, 5.02; N, 13.95. Found: C,5971; H, 4.90; N, 13.90.
2.2. Synthesis of Thiazole and Thiophene Derivatives as Coupler Compounds
- 4-aryl-2-aminothiazoles 4a-m and 4-aryl-3- substituted -2- aminothiophens 5a-g are used as starting materials for synthesis of the corresponding bishetaryl monoazo dyes 6 and 7 according to the described method in the literature [41-43]. General preparative procedures for synthesis of bishetaryl monoazo dyes 6 and 7Thiophene 3 (0.02mol) was dissolved in a mixture of acetic acid and propionic acid (12ml, ratio 5:1) and stirred for 20mins. The solution was added gradually in portion to a cold solution of sodium nitrite (1.38g, 0.02 mol) in the presence of conc. Sulfuric acid (5ml.) The diazo liquor was stirred for further 1 hr. at temperature 5-10 °C to give a clear solution, and then slowly added to cold solution of 4a-m and/or 5a-g in water (50ml) and acetic acid (10 ml) with continues stirrer. The mixture was stirred further for 2-3 hrs., neutralized with aqueous sodium hydroxide or sodium acetate. The pH value was maintained at 4-5. There resulting solid was filtered, washed with water, and dried. The crude product was recrystallized from dimethylformamide-ethanol to give violet needles in very good yield. All the other dyes 6b-6m and 7a-f were prepared in a similar manner.5-((2-amino-4-phenylthiazol-5-yl)diazenyl)-4-cyano-N-(4-ethoxyphenyl)-2-methylthiophene-3-carboxamide(6a)The dye 6a prepared in 72% yield as blue black crystals; mp. 240-2°C. IR (KBr) νmax./cm-1: 3425.9, 3328.9, 3301.3 (NH/NH2), 2926.2 (CH), 2216.7 (CN), 1623.8 (CO), 1401.411 (N=N). 1H NMR (DMSO-d6) : 1.38 (t,3H, -OCH2CH3), 2.39 (s,3H, -CH3), 4.10 (q,2H, -OCH2CH3), 6.90-8.47 (m,11,H, ArH's,NH2), 9.40 (br,1H,NH; exchangeable with D2O). MS (70eV) m/z (rel. intensity) of: 488 (20.74), 64 (100.0), 53 (11.04). Anal. Calc. for C24H20N6O2S2(488.11): C, 58.90; H, 4.85; N, 17.95. Found: C,58.71; H, 4.80; N, 17.70.Ethyl2-((2-amino-4-phenylthiazol-5-yl)diazenyl)-4-((4-ethoxyphenyl)carbamoyl)-5-methylthiophene-3-carboxylate (6b)The dye 6b prepared in 82% yield as bluish violet crystals; mp. 288-290°C. IR (KBr) νmax./cm-1 at 3426, 3328.9, 3301.3 (NH/NH2), 2978 (CH) 1640 (CO), 1417(N=N). 1H NMR (DMSO-d6) : 1.36 (t,3H, -OCH2CH3), 1.43 (t,3H, -OCH2CH3), 2.72 (s,3H, -CH3), 3.70 (br,2H,NH2), 4.27 (q,2H, -OCH2CH3), 4.35 (q,2H, -OCH2CH3), 6.90-9.45 (m,10H, ArH's,NH), Anal. Calc. for C26H25N5O4S2 (535.11): C, 58.40; H, 4.75; N, 14.95. Found: C,58.31; H, 4.70; N, 14.70.4-cyano-N-(4-ethoxyphenyl)-2-methyl-5-((4-phenyl-2-(phenylamino)thiazol-5-yl)diazenyl)thiophene-3-carboxamide (6c) The dye 6c prepared in 77% yield as dark blue crystals; mp. 452-3°C. IR (KBr) νmax./cm-1 at 3425.9 (NH), 2927.4 (CH.), 2219.7 (CN), 1661.4 (CO), 1429.0 (N=N). 1H NMR (DMSO-d6) : 1.43 (t,3H, -OCH2CH3), 2.48 (s,3H, -CH3), 4.23 (q,2H, -OCH2CH3), 6.95-8.92 (m,14,H, ArH), 9.18 (s,1H,NHph). 11.80 (br,1H,NHCO). Anal. Calc. for C30H24N6O2S2 (564.13): C, 62.40; H, 4.55; N, 14.85. Found: C, 52.31; H, 4.50; N, 14.77.Ethyl-4-((4-ethoxyphenyl)carbamoyl)-5-methyl-2-((4-phenyl-2-(phenylamino)thiazol-5-yl)diazenyl)thiophene-3-carboxylate(6d)The dye 6d prepared in 76% yield as brown crystals; mp. 292-3°C. IR (KBr) νmax./cm-1 at 3423.0 (NH), 2984 (CH), 1712, 1623.3 (CO), 1411 (N=N). Anal. Calc. for C32H29N5O4S2(611.13): C, 62.40; H, 4.56; N, 14.85. Found: C,52.31; H, 4.50; N, 14.78.5-((2-amino-4-(4-chlorophenyl)thiazol-5-yl)diazenyl)-4-cyano-N-(4-ethoxyphenyl)-2-methylthiophene-3-carboxamide(6e)The dye 6e prepared in 63% yield as blue crystals; mp. 278-280°C. IR (KBr) νmax./cm-1 at 3425.0, 3328, 3222 (NH/NH2), 2975.6 (CH), 2224.5 (CN), 1658.5 (CO), 1431.89(N=N). 1H NMR (DMSO-d6) : 1.42 (t,3H, -OCH2CH3), 2.46 (s,3H, -CH3), 4.20 (q,2H, -OCH2CH3), 6.96-8.80 (m,8,H, ArH's), 10.20 (s,2H,NH2). 11.40 (br,1H,NH). MS (70eV) m/z (rel. intensity) of (M+): 522 (49.06), 86 (100.0), 53 (72.64). Anal. Calc. for C24H19ClN6O2S2(522.10): C, 56.12; H, 4.66; N, 16.85. Found: C,52.31; H, 4.50; N, 16.78.ethyl-2-((2-amino-4-(4-chlorophenyl)thiazol-5-yl)diazenyl)-4-((4 ethoxyphenyl)carbamoyl)-5-methylthiophene-3- carboxylate( 6f) The dye 6f prepared in 77% yield as blue crystals; mp. 288-9°C. IR (KBr) νmax./cm-1 at 3423.0 (NH), 2924.5 (CH), 2221.6 (CN), 1663.3 (CO), 1404.9 (N=N). MS (70eV) m/z (rel. intensity) of (M+): 598 (17.08), 93(100.0), 53 (15.8). Anal. Calc. for C30H23ClN6O2S2(598.10): C, 60.12; H, 3.86; N, 14.85. Found: C,60.01; H, 3.50; N, 16.78.ethyl-2-((2-amino-4-(4-chlorophenyl)thiazol-5-yl)diazenyl)-4-((4 ethoxyphenyl)carbamoyl)-5-methylthiophene-3- carboxylate (6g) The dye 6g prepared in 68% yield as yellowish grey crystals; mp. 282-4°C. IR (KBr) νmax./cm-1 at 3424.0, 3376.8, 3366.3 (NH/NH2), 2930 (CH) 1640 (CO), 1430 (N=N). Anal. Calc. for C26H24ClN5O4S2(569.10): C, 55.12; H, 3.86; N, 14.85. Found: C,55.01; H, 3.50; N, 16.78.5-((4-(4-chlorophenyl)-2-(phenylamino)thiazol-5-yl)diazenyl)-4-cyano-N-(4-ethoxyphenyl)-2-methylthiophene-3-carboxamide(6h)The dye 6h prepared in 78% yield as light blue crystals; mp. < 300°C. IR (KBr) νmax./cm-1 at 3423.0 (NH), 2984 (CH), 1712, 1625.3 (CO), 1411 (N=N). Anal. Calc. for C32H28 ClN5O4S2(645.13): C, 62.40; H, 4.56; N, 14.85. Found: C,52.31; H, 4.50; N, 14.78.5-((2-amino-4-(4-chlorophenyl)thiazol-5-yl)diazenyl)-4-cyano-N-(4-ethoxyphenyl)-2-methylthiophene-3- carboxamide(6i) The dye 6i prepared in 67% yield as reddish violte crystals; mp. 278-9°C. IR (KBr) νmax./cm-1 at 3423.0, 3328, 3222 (NH/NH2), 2930.3 (CH.), 2222.6 (CN), 1653.7 (CO), 1432.9 (N=N). 1H NMR (DMSO-d6) showed signals at δ: 1.41 (t 3H,-CH2CH3), 2.46 (s,3H, CH3), 2.51(s,3H, CH3), 6.91-7.37(m, 8H, ArH's), 8.47 (s, 2H, NH2), 9.20 (s,1H, -NH-). MS m/z (rel. intensity) of: 503 (M+1; 56), 365 (100), 292 (22), 251(23), 190 (72), 135 (74), 63 (76). Anal. Calc. for C25H22N6O2S2(502.13): C, 60.40; H, 4.56; N, 14.85. Found: C,52.31; H, 4.50; N, 14.78.ethyl-2-((2-amino-4-(4-chlorophenyl)thiazol-5-yl)diazenyl)-4-((4-ethoxyphenyl)carbamoyl)-5-methylthiophene-3-carboxylate(6j)The dye 6j prepared in 71% yield as reddish violte crystals; mp. 288-9°C. IR (KBr) νmax./cm-1 at 3423.0, 3376.75,3345, ( NH/NH2) 2986 (CH.), 1709, 1666.3 (CO), 1430 (N=N). 1H NMR (DMSO-d6) showed signals at : 1.36 (t,3H, -OCH2CH3), 1.44 (t,3H, -OCH2CH3), 2.34 (s,3H, -CH3), 2.71 (s,3H, -CH3), 3.42 (br,2H,NH2), 4.27 (q,2H, -OCH2CH3), 4.35 (q,2H, -OCH2CH3), 6.89-9.40 (m,9H, ArH's, NH), Anal. Calc. for C27H27N5O4S2 (549.13): C, 59.40; H, 4.96; N, 14.80. Found: C, 58.31; H, 4.90; N, 14.78.5-((4-(4-chlorophenyl)-2-(phenylamino)thiazol-5-yl)diazenyl)-4-cyano-N-(4-ethoxyphenyl)-2-methylthiophene-3-carboxamide(6k)The dye 6k prepared in 69% yield as blueish crystals; mp. 223-4°C. IR (KBr) νmax./cm-1 at 3438.0 (NH), 2981 (CH),2210 (CN), 1633.3 (CO), 1417 (N=N). MS (70eV) m/z (rel. intensity) of: 578 (61.95), 139 (100.0), 66 (16.9). Anal. Calc. for C31H26N6O2S2 (578.16): C, 62.40; H, 4.86; N, 14.85. Found: C,59.31; H, 4.80; N, 14.78.Ethyl-2-((4-(4-chlorophenyl)-2-(phenylamino)thiazol-5-yl)diazenyl)-4-((4-ethoxyphenyl)carbamoyl)-5-methylthiophene-3-carboxylate(6l)The dye 6l prepared in 69% yield as blueish violte crystals; mp. 263-4°C. IR (KBr) νmax./cm-1 at 3430 (NH), 2978 (CH),1702,1604 (CO),1412 (N=N). 1H NMR (DMSO-d6) showed signals at : 1.43 (t,3H, -OCH2CH3), 1.33 (t,3H, -OCH2CH3), 2.36 (s,3H, -CH3), 2.52 (s,3H, -CH3), 9.35 (s,1H,NH), 11.40 (br,1H,NH), 4.25 (q,2H, -OCH2CH3), 4.39 (q,2H, -OCH2CH3), 6.95-8.19 (m,9H, ArH's,NH),. Anal. Calc. for C33H31N5O4S2(625.18): C, 63.35; H, 4.86; N, 14.95. Found: C, 59.31; H, 4.80; N, 14.88.4-cyano-N-(4-ethoxyphenyl)-2-methyl-5-((2-((4-nitrophenyl)amino)-4-phenylthiazol-5-yl)diazenyl)thiophene-3-carboxamide (6m)The dye 6m prepared in 66% yield as blue crystals; mp. 293-4°C. IR (KBr) νmax./cm-1 at 3420 (NH), 2924 (CH.), 2224 (CN), 1623.3 (CO), 1406 (N=N). 1H NMR ( DMSO-d6) showed signals at δ: 1.41 (t 3H, ,-CH2CH3), 2.46 (s,3H, CH3), 2.51(s,3H, CH3), 6.91-7.37(m,8H, ArH's), 8.47 (s,2H, NH2), 9.20 (s 1H, ,NH). Anal. Calc. for C30H23N7O4S2 (609.13): C, 64.40; H, 4.76; N, 14.95. Found: C,59.31; H, 4.50; N, 14.88.((5-amino-4-cyano-3-methylthiophen-2-yl)diazenyl)-4-cyano-N-(4-ethoxyphenyl)-2-methylthiophene-3-carboxamide(7a)The dye 7a prepared in 76% yield as greyish brown crystals; mp. 239-240°C. IR (KBr) νmax./cm-1 at 3425.7, 3382.32 , 3311.18 (NH/NH2), 2924.52 (CH.), 2210.99 (CN), 1644.77 (CO), 1437.89 (N=N). 1H NMR (DMSO-d6) showed signals at : 1.40 (t,3H, -OCH2CH3), 2.42 (s,3H, -CH3), 2.63 (s,3H, -CH3), (br,2H,NH2), 4.23 (q,2H, -OCH2CH3), 4.35 (q,2H, -OCH2CH3), 7.29-7.62 (dd,4H,AB system ArH's), 9.59 (s,1H,NH). Anal. Calc. for C21H18N6O2S2 (450.13): C, 61.40; H, 4.73; N, 14.95. Found: C,59.31; H, 4.50; N, 14.88.Ethyl-2-((5-amino-4-cyano-3-phenylthiophen-2-yl)diazenyl)-4-((4-ethoxyphenyl)carbamoyl)-5-methylthiophene-3-carboxylate(7b)The dye 7b prepared in 71% yield as brown crystals; mp. 251-2°C. IR (KBr) νmax./cm-1 at 3382 (NH), 2926 (CH.), 2212 (CN), 1644 (CO), 1402 (N=N). 1H NMR (DMSO-d6) showed signals at : 1.42 (t,3H, -OCH2CH3), 2.49 (s,3H, -CH3), 3.77 (br,2H,NH2), 4.24 (q,2H, -OCH2CH3), 6.91-9.10 (m,9H, ArH's,) 9.45 (br, 1H, NH). Anal. Calc. for C26H20N6O2S2 (512.15): C, 60.40; H, 4.75; N, 14.98. Found: C,59.31; H, 4.50; N, 14.85.ethyl-2-amino-5-((3-cyano-4-((4-ethoxyphenyl)carbamoyl)-5-methylthiophen-2-yl)diazenyl)-4-phenylthiophene-3-carboxylate(7c)The dye 7c prepared in 60% yield as blue crystals; mp. 284-6°C. IR (KBr) νmax./cm-1 at 3420.14, 3410.20, 3289.96 (NH/NH2), 2979.48 (CH.), 2218.70 (CN), 1668.12(CO), 1431.89 (N=N). Anal. Calc. for C28H25N5O4S2 (559.13): C, 60.45; H, 4.77; N, 14.96. Found: C,59.41; H, 4.55; N, 14.85.ethyl-2-((5-amino-3-(4-chlorophenyl)-4-cyanothiophen-2-yl)diazenyl)-4-((4-ethoxyphenyl)carbamoyl)-5-methylthiophene-3-carboxylate(7d)The dye 7d prepared in74% yield as light blue crystals; mp. 266°C. IR (KBr) νmax./cm-1 at 3422.06, 3388.32, 3311.18 (NH/NH2), 2975.52 (CH.), 2213.99 (CN), 1655.77 (CO), 1431.89 (N=N). MS m/z (rel. intensity) of: 546 (M+1; 56), 380 (100), 292 (22), 251(23), 190 (72), 135 (74), 63 (76). Anal. Calc. for C26H19ClN6O2S2 (546.06): C, 60.65; H, 4.87; N, 14.90. Found: C,59.45; H, 4.65; N, 14.81.ethyl-2-amino-4-(4-chlorophenyl)-5-((3-cyano-4-((4- ethoxyphenyl)carbamoyl)-5-methylthiophen-2-yl)diazenyl) thiophene-3-carboxylate(7e)The dye 7e prepared in74% yield as light blue crystals; mp. 266°C. IR (KBr) νmax./cm-1 at 3416 (NH), 3312.14, 3196.43 (-NH2), 2922.59 (-CH-.), 2211.95 (CN), 1627.63 (CO), 1406.82 (N=N). Anal. Calc. for C29H27ClN5O4S2 (573.06): C, 60.65; H, 4.87; N, 14.90. Found: C,59.45; H, 4.65; N, 14.81.5-((5-amino-3-(4-chlorophenyl)-4-cyanothiophen-2-yl)diazenyl)-4-cyano-N-(4-ethoxyphenyl)-2-methylthiophene-3-carboxamide(7f)The dye 7f prepared in 81% yield as blue crystals; mp. 259°C. IR (KBr) νmax./cm-1 at 3422.06, 3378.32, 3311.18 (NH/NH2), 2924.52 (CH.), 2210.99 (CN), 1650.77 (CO), 1431.89 (N=N). Anal. Calc. for C27H22N6O2S2 (562.06): C, 60.55; H, 4.82; N, 14.93. Found: C, 59.65; H, 4.69; N, 14.86.ethyl-2-amino-4-(4-chlorophenyl)-5-((3-(ethoxycarbonyl)-4-((4-ethoxyphenyl)carbamoyl)-5-methylthiophen-2-yl)diazenyl)thiophene-3-carboxylate(7g)The dye 7g prepared in 67% yield as blue crystals; mp. 195°C. IR (KBr) νmax./cm-1 at 3423.9 (NH / NH2), 2978.1 (CH.), 2217.0 (CN), 1731,1658.2 (CO), 1433.1 (N=N). 1H NMR spectrum of compound (7e DMSO-d6) showed signals at : 0.91 (t,3H, -OCH2CH3), 1.43 (t,3H, -OCH2CH3), 2.34 (s,3H, -CH3), 2.49 (s,3H, -CH3), 4.02 (q,2H, -OCH2CH3), 4.27 (q,2H, -OCH2CH3), 6.89-8.20 (m,11H, ArH's,NH2), 9.25(s,1H,NH). Anal. Calc. for C29H27N5O4S2 (573.16): C, 60.55; H, 4.77; N, 14.90. Found: C,59.45; H, 4.65; N, 14.82.
2.3. Dyeing and Fastness Determinations
2.3.1. Preparation of Dye Dispersion
- The required amount to the dye (2%) was dissolved in suitable solvent and added dropwise with stirring to a solution of Basojet (2 g/l), an anionic dispersing agent of BASF, then the dye was precipitated in a fine dispersion ready for use dyeing.
2.3.2. Dyeing Procedure
- The fabric was dyed with 2% dye (calculated on weight the fabric), 1% Basojet (as dispersing agent), kept at a liquor ratio of 20:1. The process was started at 60°C.; the temperature was then raised to 130°C over 30 minutes and maintained there for 1 hour. After cooling, the fabrics were taken out and treated with a solution of 2% sodium bisulphate, 2% sodium hydroxide, and 0.1% Basojet at 70°C for 30 minutes. Finally, the fabrics were rinsed and dried at 60°C.The dye bath (1:20, good to dye liquor ratio) Additional dispersing agent (0.5-1.0 g/l) was added and the pH of the bath adjusted to 5.5 using glacial acetic acid. Dyeing carried out by raising the dye bath temperature from 20 to 130°C at a rate of 3°C/min and holding at this temperature for 60 minutes before rapidly cooling to 50°C at 9.9°C/min. The dyed fibers was then rinsed with cold water, reduction-cleared using sodium hydroxide (2 g/l) and sodium hydrosulphite (1 g/l) and soaped with 2% nonionic detergent and ammonia (pH 8.5) at 50°C for 30 minutes to improve washing fastness.
2.3.3. Colour Fastness Testing
2.3.3.1. Color Measuremets
- The color of dyed polyester fabrics was assessed quantitatively using continuous scanning for reflectance color measurements in the visible spectrum (390 – 700 nm), hue angle (H), chromaticity difference (ΔC) and color spectrocolourimeter build-up (K/S), and total colour difference (ΔE).
2.3.3.2. Washing Fastness
- A specimen of dyed polyester sample was stitched between two pieces of undyed cotton and polyester fabrics (10 cm × 4 cm), all approximately of equal weight and then washed at 50°C. The staining on the white adjacent fabrics was assessed according to the international Grey scale [16, 17], where 1 = poor, 2 = fair, 3 = moderate, 4 = good, 5 = excellent. The AATCC standard test method 15 –1960 was used. The acid solution (pH = 3.5) contained sodium chloride (10 g/l), lactic acid U.S.P 85% (1g/l), disodium orthophosphate anhydrous (1 g/l) and histidine monohydrochloride (0.25 g/l). The alkaline solution contained sodium chloride (10 g/l), ammonium carbonate (4 g/l), disodium orthophosphate anhydrous (1 g/l) and histidine monohydrochloride (0.25 g/l). A composite specimen was made from the dyed sample as a layer between undyed cotton and polyester fabrics as the same weight as the dyed sample, composite specimen was immersed in the perspiration solution for 30 minutes with occasional agitation and squeezing to insure complete wetting, then stitched between the plastic in such a way that the dyed sample will be in a vertical position when placed in the oven. The loaded sample was kept in an oven at 37°C for 6–8 hours, so that fibers were dried by conventional means. The change in color of the dyed material and staining on the undyed adjacent fabrics was assessed according to the international Grey scale.
2.3.3.3. Fastness to Rubbing
- The dyed polyester fiber was placed on the base of crockmeter (Atlas electronic type), so that it rested flat on the abrasive cloth with its long dimension in the direction of rubbing. A square of white testing cloth was mount over the end of the finger which protects downward on the dry specimen sliding back and forth twenty times by making ten complete turns of the crank at the rate of one turn per second. For wet rubbing test, the testing squares were thoroughly wet in distilled water and squeezed between filter papers through hand wringer under standard conditions. The rest of the procedure is the same as the dry crocking test. The staining on the white cloth was assessed according to the international Grey scale.
2.3.3.4. Fastness to Sublimation
- The fastness to sublimation was assessed according to AATTC 107-1999 test. The dyed polyester fiber was stitched between two pieces of white polyester and cotton fabrics, all of equal length. The samples were treated at 185°C and 210°C for 30 seconds. After conditioning the sample for 16 hours, the change in color of the dyed sample and the staining of white ones were assessed according to the Grey scale.
2.3.3.5. Fastness to Light
- The tested samples and standard blue scales were ex-posed to the “Weather-o-meter” (Atlas Electric Devices Co. USA) [44, 45]. The exposure of both was discontinued at one of the time indicated in AATCC standard of 5, 10, 20, 40, 80, 160, 320 and 640 hours, at which it shows just appreciable fading. In the present work the dyed fabrics were exposed to light for 40 hours, after which the fibers were allowed to lie in the dark at room temperature for about two hours in order to cool-off and regain normal moisture from air. The samples were viewed in the daylight fluorescent lamp. The changes in color were assessed according to the following scale ratings; (1-poor, 3-moderate, 5-good, and 8- very good) standard of AATCC.
3. Results and Discussion
- In this study, aminothiphene intermediates 3a,b were prepared using Gewalds methodology through the reaction outlined in Scheme 1. This convenient methodology includes the condensation of the 1,3-dicarbonyl compounds, p-acetophenetidide with the activated nitriles such as malononitrile, ethylcyanoacetate in the presence of sulfur in ethanol/basic media.
 | Scheme 1 |
3.1. Preparation of Dyes 6 and 7
- Synthesis of bishetaryl monoazo dyes 6 and 7 is shown in Scheme 2. Thiophene amines were diazotized using sodium nitrite in the presence of sulfuric acid, followed by coupling with 4-aryl-2-aminothiazoles 4a-4h and 4-aryl-3-substituted -2-aminothiophens 5a-5f.
 | Scheme 2 |
3.2. Molecular Modelling Studies
- In trying to achieve better insight into the molecular structure of the most preferentially steroisomer and\or tautomeric forms for compounds 3a,b and 5a-m the conformational analysis of the target compounds has been performed using the MMFF94 force-field [46] (calculations in vacuo, bond dipole option for electrostatics, Polake Ribiere algorithm, RMS gradient of 0.01 kcal/A mol) implemented in MOE [47]. The most stable conformer was fully geometrical optimized with PM3 molecular orbital with semi-empirical Hamiltonian molecular orbital calculation MOPAC 7 package (Fig1,2). The lowest minimization energy for the 6a-m and 7a-m exhibited a common feature:The higher HOMO energy values show the molecule is a good electron donor, in other hand, the lower HOMO energy values indicate that, a weaker ability of the molecules for donating electron. LUMO energy presents the ability of a molecule for receiving electron [48].The thiazole rings stabilized by planner mode with each other, which stabilized with phenyl rings in coplanar position in all thiazole dyes (6a-m) (fig 1).
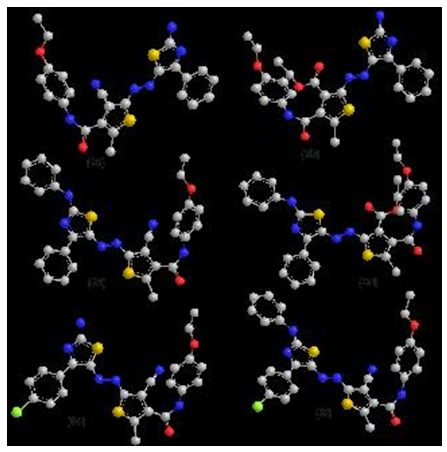 | Figure 1. Lowest minimization energy for represented sample for thiazole dye at PM3 method |
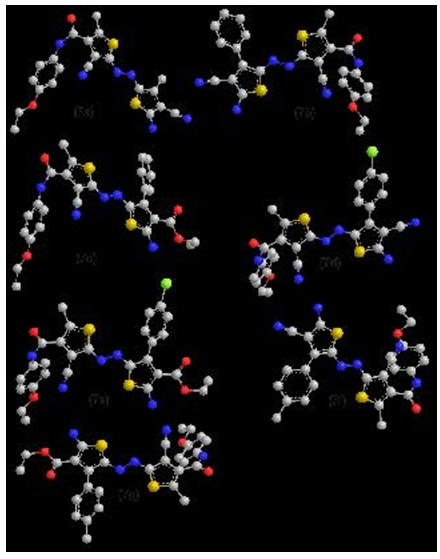 | Figure 2. Lowest minimization energy for thiophene dye at PM3 method |
3.3. Absorption Spectra
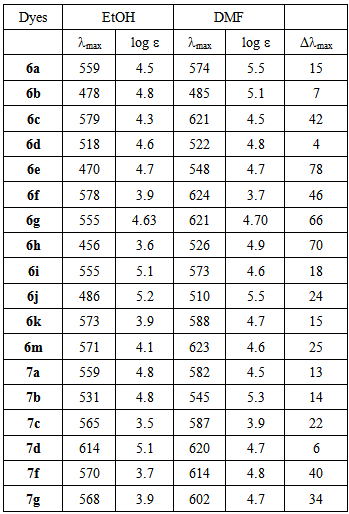
- Absorption spectra of the azo dyes derived from aminothiophene derivatives were recorded in solvents with different dielectric constants, and the results are summarized in Table 1. The colour of these azo dyes depends on the nature of both the diazo and the coupling components.
|
 types).The introduction of the cyano group as an electron withdrawing subsituent onto the thiophene ring produces a bathochromic shift of the absorption band, while the replacement of the cyano group by an ethyl ester group exerts a notable hypsochromic shift. By the way, the absorption maxima of dye 6a shifted apparently in Ethanol bigger 81nm than that of dye 6b, but in dimethylforamide shifted increase 89 nm. This was observed for the azo dye 6a where the introduction of the cyano group as an electron-acceptor results in a bathochromic shift of 81 nm, as compared with the ethyl ester group in the diazo component of azo dye 6b. Similar result is also observed for the azo dyes pairs 6c and 6d, 6f and 6g, 6i and 6j, 7f and 7g. This is attributed to more extensive electron delocalization and the smaller steric requirements of the rod-like cyano group [49]. Bathochromic shifts of the visible absorption band were also observed on increasing the solvent polarity as in seen in Table 1, where a difference of 4-78 nm in λmax. was noticed upon measuring the azo dyes in ethanol and DMF. This is expected for a system in which the excited state is more polar than the ground state [50, 51]. The data in Table reveal that the change in the λmax. value depends on the nature of the coupling site as well as the nature of the substituents at the terminal amino group. Bathochromic shifts were observed on replacing hydrogen atom in 2-amino thiazol by phenyl ring where a difference of 18 - 108 nm in λmax. was seen upon comparing the azo dyes pairs 6a and 6c, and 6b and 6d, and 6e and If, and 6i and 6k, using the same solvent. It was also observed from Table 1 that dyes 6d, 6e, 6i and 6j possessing a thiazole coupler, absorb hypsochromically compared to the dyes 7c,7d, 7f and 7g involving a thiophene coupler, in different solvent.
types).The introduction of the cyano group as an electron withdrawing subsituent onto the thiophene ring produces a bathochromic shift of the absorption band, while the replacement of the cyano group by an ethyl ester group exerts a notable hypsochromic shift. By the way, the absorption maxima of dye 6a shifted apparently in Ethanol bigger 81nm than that of dye 6b, but in dimethylforamide shifted increase 89 nm. This was observed for the azo dye 6a where the introduction of the cyano group as an electron-acceptor results in a bathochromic shift of 81 nm, as compared with the ethyl ester group in the diazo component of azo dye 6b. Similar result is also observed for the azo dyes pairs 6c and 6d, 6f and 6g, 6i and 6j, 7f and 7g. This is attributed to more extensive electron delocalization and the smaller steric requirements of the rod-like cyano group [49]. Bathochromic shifts of the visible absorption band were also observed on increasing the solvent polarity as in seen in Table 1, where a difference of 4-78 nm in λmax. was noticed upon measuring the azo dyes in ethanol and DMF. This is expected for a system in which the excited state is more polar than the ground state [50, 51]. The data in Table reveal that the change in the λmax. value depends on the nature of the coupling site as well as the nature of the substituents at the terminal amino group. Bathochromic shifts were observed on replacing hydrogen atom in 2-amino thiazol by phenyl ring where a difference of 18 - 108 nm in λmax. was seen upon comparing the azo dyes pairs 6a and 6c, and 6b and 6d, and 6e and If, and 6i and 6k, using the same solvent. It was also observed from Table 1 that dyes 6d, 6e, 6i and 6j possessing a thiazole coupler, absorb hypsochromically compared to the dyes 7c,7d, 7f and 7g involving a thiophene coupler, in different solvent.3.4. Analysis of Dyeing Performance from Aqueous Dispersions
- This work describes the application of the synthesized compounds as new disperse dyes for dyeing polyester fibers where a range of bright color shades has been obtained as the visual color shades varied from Grey, violet, move, reddish, dark red to blue. Generally, variation in color of these dyes results from the alternation in the coupling components. The performance is further examined by means of the following techniques.
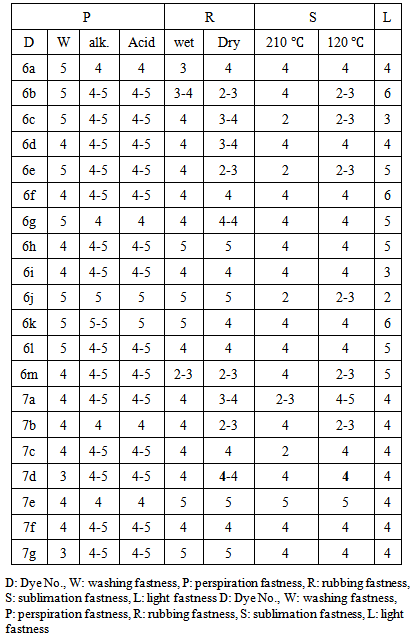
3.5. Color Fastness Evaluation
- The synthesized disperse dyes under investigation were applied to polyester fibers at 2% shade by high temperature- pressure technique (130℃), where they produced generally deep and bright hues, ranging from grey to blue. The dyes on polyester fibers were evaluated in terms of their fastness properties as shown in table 2.
|
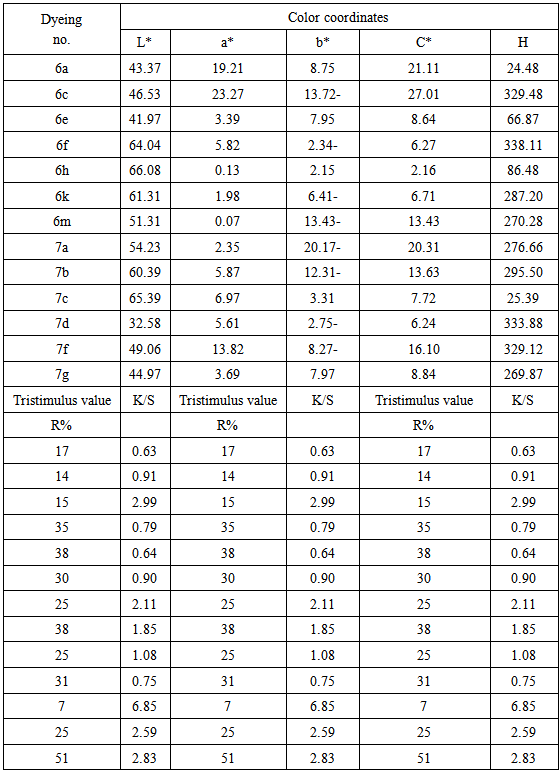
3.6. Color Difference Measurements
- The color parameters of the dyed polyester fibers were measured using the GretagMacbeth CE 7000a spectropho tometer and showed in table 3. The assessment of color-dyed fibers was made in terms of tristimulus colorimetry.The following CIELAB coordinates are measured, lightness (L*), chroma (C*), hue angle from 0º to 360º (h), (a*) value represents the degree of redness (positive) and greenness (negative) and (b*) represents the degree of yellowness (positive) and blueness (negative). A reflectance spectrophotometer (GretagMacbeth CE 7000a) was used for the colorimetric measurements on the dyed samples. K/S value given by the reflectance spectrometer is directly cor-related with the dye concentration on the dye substrate ac-cording to the Kubelka–Munk equation: K/S = (1-R)2/2R, where K = absorbance coefficient, S = scattering coefficient, R = reflectance ratio.
|
4. Conclusions
- Thienyl-2-azo and thienyl-5-azo disperse dyes have been synthesized and their spectral properties investigated. The nature of the coupling components as well as the solvent polarity afforded a bathochromic shift with aminothiophene. The absorption maxima of the investigated azo dyes showed larger bathochromic shifts in DMF than in Ethanol solvent. All of them were investigated for their dyeing characteristics on polyester fibers and showed good affinity to polyester fibers. These results are in line with the previously reported by Műller [52] on the effect of substituent in the dye structure and hue. The electronic absorption spectra give bright hues from violet to blue on polyester fibres.The dyed fibres exhibit very good to excellent washing, perspiration and sublimation fastness properties with little variation in the good to excellent rubbing fastness. The remarkable degree of levelness and brightness after washing is indicative of good penetration and the excellent affinity of these dyes for polyester fiber. This in combination with the ease of preparation makes them particularly valuable.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML