-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Textile Science
p-ISSN: 2325-0119 e-ISSN: 2325-0100
2015; 4(4): 84-96
doi:10.5923/j.textile.20150404.03
Water- and Oil-Repellency Properties of Cotton Fabric Treated with Silane, Zr, Ti based Nanosols
Nurhan Onar1, Gulfem Mete1, Aysun Aksit2, Bengi Kutlu2, Erdal Celik3, 4, 5
1Pamukkale University, Engineering Faculty, Department of Textile Engineering, Denizli, Turkey
2Dokuz Eylul University, Engineering Faculty, Department of Textile Engineering, Izmir, Turkey
3Dokuz Eylul University, Engineering Faculty, Department of Metallurgical and Materials Engineering, Izmir, Turkey
4Dokuz Eylul University, Engineering Faculty, Department of Nanoscience and Nanoengineering, Izmir, Turkey
5Dokuz Eylul University, Engineering Faculty, Center for Production and Applications of Electronic Materials, Izmir, Turkey
Correspondence to: Nurhan Onar, Pamukkale University, Engineering Faculty, Department of Textile Engineering, Denizli, Turkey.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Water- and oil-repellent cotton fabricwas developed by sol-gel technique in this study. In order to achieve this, silane-, Zr- and Ti-based nanosols were prepared using tridecafluorooctyltriethoxysilane, hexadecyltrimethoxysilane, vinyltrimethoxysilane, phenyltriethoxysilane, 3-aminopropyl trimethoxysilane, glycidyloxypropyltriethoxysilane, zirconium (IV) acetylacetonate and titanium (IV) isopropoxide as precursors. The fabric samples were treated with nanosols using pad-dry-cure processes. The structural and microstructural nature of the coatings was characterized by using Fourier transform infrared spectroscopy, X-ray diffractometry and scanning electron microscopy. In addition, water- and oil repellency properties, contact angle and the wash fastness of the coated fabric samples were determined. It was found that the cotton fabrics with the best water- and oil-repellency properties could be produced using nanosols containing hexadecyltrimethoxysilane as a precursor and Guard AFB as a commercial water-oil repellent agent with a lower concentration than that of conventional process. Moreover, the natural textile performance properties of the fabric samples did not significantly change after treatment with the nanosols.
Keywords: Water-Oil repellency, Surface modification, Sol-Gel technology, Contact angle, Finishing
Cite this paper: Nurhan Onar, Gulfem Mete, Aysun Aksit, Bengi Kutlu, Erdal Celik, Water- and Oil-Repellency Properties of Cotton Fabric Treated with Silane, Zr, Ti based Nanosols, International Journal of Textile Science, Vol. 4 No. 4, 2015, pp. 84-96. doi: 10.5923/j.textile.20150404.03.
Article Outline
1. Introduction
- Sol-gel technology has recently been attracting interest in connection with its various functional properties, including those of UV protectivity, antistatic, antimicrobial, self-cleaning, crease recovery, superhydrophobicity, water, oil and soil-repellency and flame retardancy, in relation to textile materials. There are many researches about enhancing the efficiency of the water/oil repellent agents currently used and developing novel methods such as plasma, sol-gel, graft polymerization and magnetron sputtering to create superhydrophobic surfaces. Fluorocarbon compounds, silicone compounds, resins based on melamine and urea derivatives, and paraffin emulsion containing zirconium and aluminium were applied by pad-dry-cure method for conventional water- and oil-repellent cotton fabric. A nanotechnological sol-gel process could be alternatively presented as a durable washing water- and oil-repellent finishing process using fewer chemical agents and without using hazardous and toxicological chemicals such as fluorocarbon-based chemicals for textile materials [1-6].Many studies have reported on the use of perfluorooctyltriethoxysilane monomer to develop water/oil-repellent textile materials by using a sol-gel process [7-14]. Yu et al. carried out complex superhydrophobic coatings on cotton fabrics with contact angle values between 133o and 145o by using perfluorooctylatedquarternary ammonium silane- and silica nanoparticle-based nanosols [3]. There are also some researches using polymeric fluorine compounds such as Nafion to produce water/oil-repellent textile materials in literature [15, 16]. Fabbri et al. produced hydrophobic and oleophobic surfaces using tetraethylorthosilicate (TEOS) as a precursor and a perfluoropolyether oligomer as a material with low free surface energy [17-19]. Researchers found that the addition of small quantities alkyltrialkoxysilanes in silica sols caused a significant hydrophobic effect on textile materials [1, 2, 20, 21]. When alkyltrialkoxysilanes with an increasing chain length were used, an increasing chain length up to 12 carbons resulted in decreasing water uptake. The usage of alkyltrialkoxysilanes with longer alkyl chains did not decrease water uptake further [22]. It was found that glass substrates coated with nanosols based on vinyltriethoxysilanes exhibited very good hydrophobicity and their critical surface tension was very low at 25 mN/cm [23]. Researchers determined that the contact angle values of polymeric foil coated with pure vinyltriethoxysilane-based nanosols were higher than 100o [24]. Textile materials were coated with nanosols containing vinyltrimethoxysilane with or without TEOS, cured and treated with UV light to start cross-linking their vinyl functional groups. Water uptake of the fabric samples reduced after exposure to UV light [25]. Textor and Mahltig researched producing hydrophobic and antistatic textile materials by coating with SiO2-based sols [26, 27]. Xue et al. created superhydrophobic surfaces of a hierarchical nature on cotton fabrics by complex coating with epoxy functionalized silica nanoparticles with a contact angle value of 170° [28]. Bae et al. treated textile materials with silica nanoparticles and commercial agent for producing superhydrophobic fabrics and obtained higher contact angle values than 130° [29]. Li et al. successfully produced superhydrophobic surfaces at higher values than 151o of contact angle by using water glass and hexadecyltrimethoxysilane as a precursor using the sol-gel method [30]. Boukhriss et al. modified the surfaces of cotton, polyester and polyamide fabrics using chloropropyltriethoxysilane as a precursor utilizing the sol-gel process to improve their water repellency, thermal stability and mechanical properties [31]. Lakshmi et al. fabricated superhydrophobic sol-gel nanocomposite coatings on glass substrate by incorporating silica nanoparticles in an acid-catalyzed sol of methyltriethoxysilane [32]. Vasiljevic et al. applied a sol mixture, which included 1H,1H,2H, 2H-perfluorooctyltriethoxysilane,3-(trimethoxysilyl)-propyldimethyloctadecyl ammonium chloride and P, P-diphenyl-N-(3-(trimethoxysilyl) propyl) phosphinic amide, to cotton fabric to prepare cotton fibres with novel multifunctional water- and oil-repellency, antibacterial and flame-retardancy properties [33]. In the present study, we aimed to obtain water- and oil-repellency properties for cotton fabrics by using the sol-gel process. To achieve this aim, we chose the precursors taking into account previous studies in literature and treated cotton fabrics by using the sol-gel process, with tetra ethylorthosilicate, tridecafluorooctyltriethoxysilane, hexadecyltrimethoxysilane, vinyltrimethoxysilane, phenyltriethoxysilane, 3-aminopropyl trimethoxysilane, glycidyloxypropyltriethoxysilane, zirconium (IV) acetylacetonate and titanium (IV) isopropoxide as precursors. Our present study included other precursors which have not been comparatively reported in literature. We first compared the effects of these precursors on the water/oil-repellency properties of cotton fabric in literature.
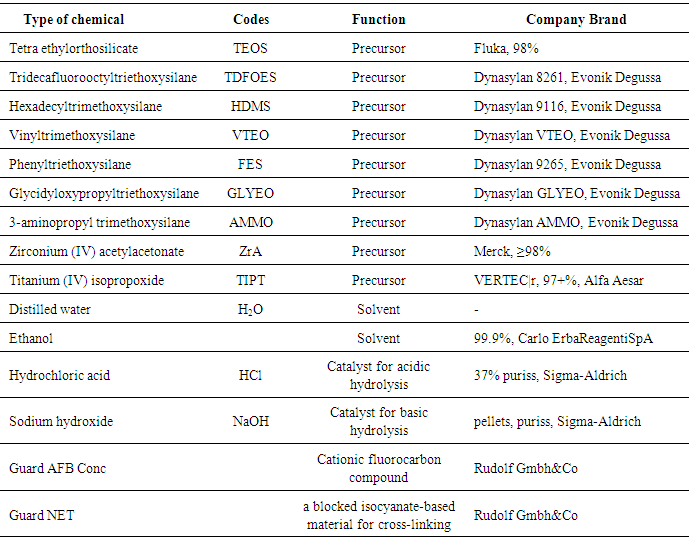
2. Materials
- Scoured and bleached 100% plain-weave cotton fabrics (weight 110 g/m2, 22 picks/cm, 32 ends/cm) were used as substrate in this research. All the chemicals used were of reagent grade and shown in Table 1. All experiments were carried out in duplicate.
|
3. Methods
3.1. Sol-gel Processing
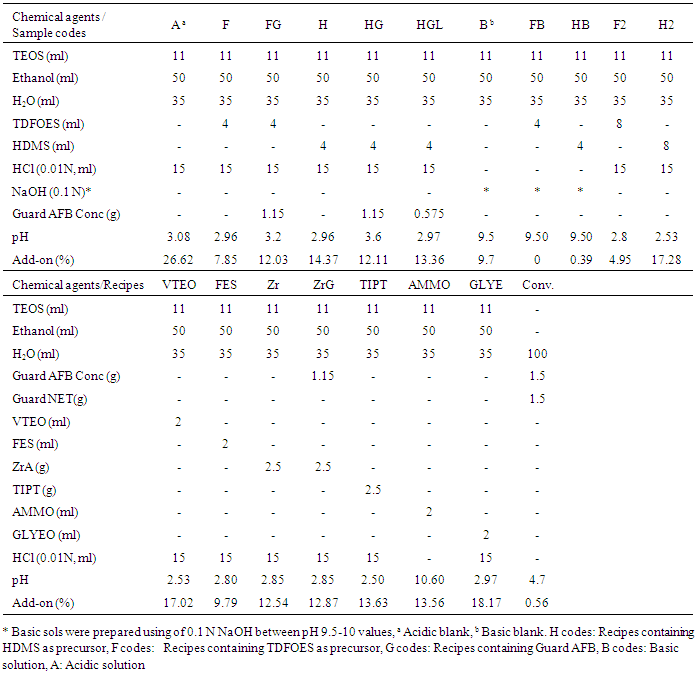
- The solutions were prepared by mixing TEOS with ethanol and then adding distilled water followed by 0.01 N HClsolution for acidic hydrolysis or 0.1 N NaOH solution for basic hydrolysis, then mixing. Next, other precursors such as fluorine or long carbon chain containing precursors or other precursors were added to the solution. The pH of the solutions was measured using a standard pH meter. These solutions were stirred at 25°C for 30 min using a magnetic stirrer. After that, transparent solutions were obtained. Novel nanosol recipes were also studied novel nanosol recipes by adding a one-third and two-thirds rate of concentration of Guard AFB (G code) to a conventional recipe in order to provide the oil repellency properties of fabric samples because the fabric samples treated with recipes containing TDFOES, HDMS and ZrA have better water-repellency properties. The used recipes, final pH values of silica sols and add-on values of the coated fabric with these sols are given in detail in Table 2. The fabrics were dried to measure their dry mass in certain conditions and then weighed. The fabrics were dipped in nanosol solutions and then padded. Then the fabrics were squeezed using a padding machine (Ataç Laboratory Machines and Control Panels, İstanbul, Turkey) at 90% of pickup and 1.6 bar of nip pressure. The padded fabrics were dried using a Nuve Dry Heat Sterilizer (Nüve Sanayi Malzemeleri İmalatve Ticaret A.Ş., Ankara, Turkey) at 100°C for 10 min and cured using a laboratory-type steamer (Ataç Laboratory Machines and Control Panels, İstanbul, Türkey) at 160°C for 1 min in air. The coating process was carried out three times and the flow chart of the sol-gel process is illustrated in Figure 1.
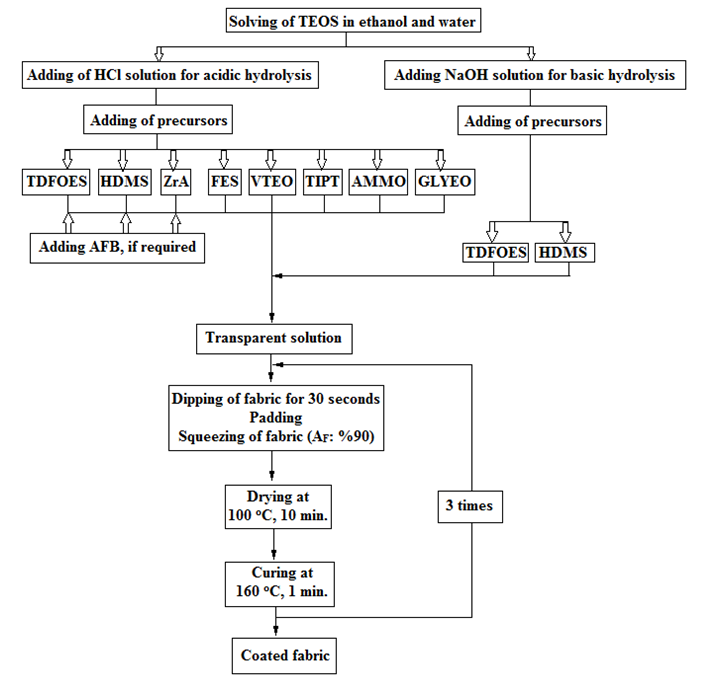 | Figure 1. Flow chart of sol-gel treatment of cotton fabric for water and oil repellency properties |
|
3.2. Conventional Processing
- A conventional water- and oil-repellent finishing process was carried out using the recipe suggested by the company in order to compare the water- and oil-repellency properties of the fabrics coated by using the conventional and the sol-gel process. The fabrics were dipped in solutions prepared as conventional recipes, padded (90% of pickup), dried at 100°C for 10 min and cured at 170°C for 3 min.
3.3. Characterization
- The pH values of nanosols were determined using pH meter. The add-on values of the treated fabric by sol-gel process and conventional process were calculated according to Equation 1[34].
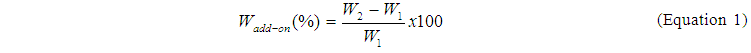 where W1 is the weight of the untreated fabric and W2 is the weight of the treated fabric. Whiteness (Stensby) of fabric samples were measured using spectrophotometer (Datacolor Applied Color Systems, Inc., USA) in UV-included, Specular excluded, USAV mod with fourfold fabric samples in D65 (daylight source) at 10o measurement angle. Bending length values of fabric samples were measured using Shirley Stiffness Tester (SDL Atlas Textile Testing Solutions, England) as BS 3356, 1992 standard. Tear strength values of fabric samples were measured using Elmatear Digital Tear Tester (James H. Heal Co. Ltd. Halifax, England) as ASTM D5035, 1995 standard. Water repellency properties of fabric samples were measured with spray test as AATCC Test Method 22 standard by water repellency tester. The contact angle of the fabrics was determined using KSV Cam 100 Instruments in order to determine the water repellency properties of fabrics. Oil repellency test for fabric samples were done using some hydrocarbon compounds as AATCC Test Method 118 standard. The wash fastness of their water-oil repellency properties was tested by washing conditions according to TS EN ISO 105- C06- A1S at 40°C without balls using Linitest plus apparatus. Washing process was repeated 5 times. Absorption spectras of treated and untreated fabric samples were measured over the range of 4000 to 650cm−1 at room temperature using Fourier transform infrared spectroscopy with attenuated total reflectance sampler (FTIR-ATR). XRD patterns of thin films on fabrics were determined to indentify phase structure by means X-ray diffractometer (XRD) at 40 kV and 36 mA with a CuKα irradiation (wavelength, λ=0.15418 nm) in the θ-2θ mode with a scan speed of 4o/min. The surface topographies of coated and uncoated fabrics were examined with the aid of scanning electron microscopy operating at 3 kV with X2000 and X1000 magnification.
where W1 is the weight of the untreated fabric and W2 is the weight of the treated fabric. Whiteness (Stensby) of fabric samples were measured using spectrophotometer (Datacolor Applied Color Systems, Inc., USA) in UV-included, Specular excluded, USAV mod with fourfold fabric samples in D65 (daylight source) at 10o measurement angle. Bending length values of fabric samples were measured using Shirley Stiffness Tester (SDL Atlas Textile Testing Solutions, England) as BS 3356, 1992 standard. Tear strength values of fabric samples were measured using Elmatear Digital Tear Tester (James H. Heal Co. Ltd. Halifax, England) as ASTM D5035, 1995 standard. Water repellency properties of fabric samples were measured with spray test as AATCC Test Method 22 standard by water repellency tester. The contact angle of the fabrics was determined using KSV Cam 100 Instruments in order to determine the water repellency properties of fabrics. Oil repellency test for fabric samples were done using some hydrocarbon compounds as AATCC Test Method 118 standard. The wash fastness of their water-oil repellency properties was tested by washing conditions according to TS EN ISO 105- C06- A1S at 40°C without balls using Linitest plus apparatus. Washing process was repeated 5 times. Absorption spectras of treated and untreated fabric samples were measured over the range of 4000 to 650cm−1 at room temperature using Fourier transform infrared spectroscopy with attenuated total reflectance sampler (FTIR-ATR). XRD patterns of thin films on fabrics were determined to indentify phase structure by means X-ray diffractometer (XRD) at 40 kV and 36 mA with a CuKα irradiation (wavelength, λ=0.15418 nm) in the θ-2θ mode with a scan speed of 4o/min. The surface topographies of coated and uncoated fabrics were examined with the aid of scanning electron microscopy operating at 3 kV with X2000 and X1000 magnification. 4. Results and Discussion
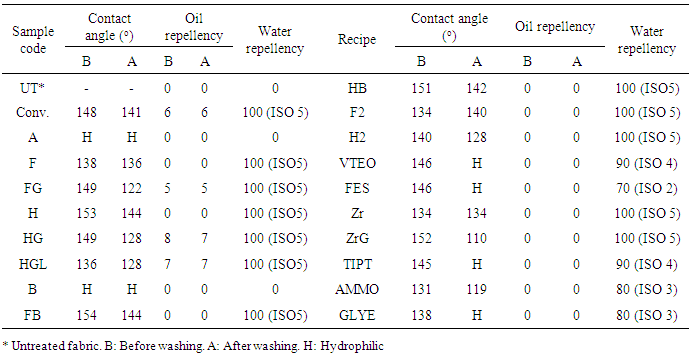
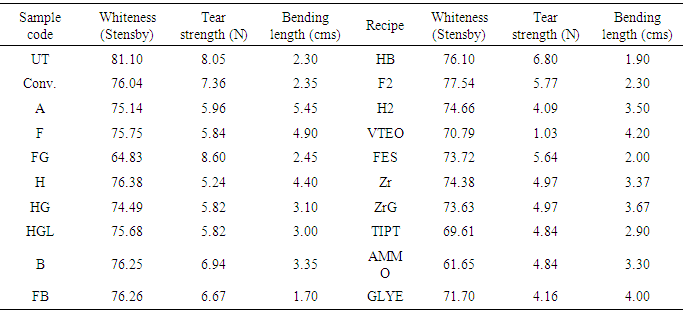
- Within the framework of the present study, water repellency, oil repellency, contact angle, whiteness, tear strength and bending length measurements were carried out to see the performance of the fabrics before and after the washing processes. Water repellency and oil repellency properties, contact angle, whiteness, tear strength and bending length values of the fabric samples treated with recipes in Table 2 before and after the washing process are given in Table 3 and Table 4.
|
|
4.1. The pH of Solutions
- The pH values of the nanosols are given in Table 2. The pH values of acidic nanosols varied from 2.5 to 3.6, whilst those of basic nanosols ranged from 9.5 to 10.6. The pH value of baths prepared as conventional recipes was 4.7. According to these results, it is clear that pH values of nanosols significantly affect the add-on values, bending length, tensile strength and tearing strength properties of the fabric samples treated with these nanosols, but not the whiteness and oil-repellency properties of the fabric samples. It is noticeable that FB samples containing TDFOES as a precursor in basic conditions have a higher contact angle, while H samples containing HDMS as a precursor in acidic conditions have a higher contact angle.Since the pH values of the solutions are an important factor influencing the formation of the polymeric three-dimensional structure of the gel during the gelation process, they should be taken into consideration when preparing solutions. While a ramified structure is randomly formed in acidic conditions, separate clusters are formed from the solutions showing basic characters as explained in Ref. [35]. Nanosols hydrolyzed under acidic conditions usually result in weakly cross-linked condensation products with more densely layered structure after coating, whereas basic catalyzed sols cause particle aggregates with larger pores [35, 6].
4.2. Add-on Values of the Fabrics
- The add-on values of the fabric samples treated with nanosols are listed in Table 2. The mass of fabrics increased after nanosol treatment. In particular, the add-on values of the fabric samples treated with nanosols in acidic conditions were higher, while those of the fabric samples treated with nanosols in basic conditions were lower (0% and 0.39%). An add-on value of 0.56% was obtained for the fabric samples treated with a conventional recipe. Note that H samples, which have the best water-repellency properties, have an add-on value of 14.37%, whilst GLYE and A samples have the highest add-on values at 26.62% and 18.17%, respectively. This may be the result of the high cross-linking properties of the GLYEO precursor. The highest add-on values among the VTEO, FES, Zr, ZrG, TIPT, AMMO and GLYE samples were obtained for the VTEO and GLYE samples (17.02% and 18.17%, respectively), while the lowest add-on value was obtained for the FES samples (9.79%).
4.3. Oil- and Water-repellency Properties
- It was found by using a spray test that fabric samples treated by nanosols have excellent water-repellency properties (90–100), while untreated fabric has hydrophilic properties as shown in Table 3. The fabric samples treated with nanosols containing TDFOES, HDMS, VTEO, ZrA and TIPT as precursors have good water-repellency properties (90–100). However, the fabric samples treated with nanosols containing FES, AMMO and GLYEO as precursors have relatively low water-repellency properties (70–80). The results were in line with the results of the contact angles of the fabric samples. The fabric samples treated with nanosols have high contact angle values (131–154o) before washing. Some samples treated with nanosols containing VTEO, FES, TIPT and GLYEO were hydrophilic after washing as five times, while the contact angles of fabric samples treated with nanosols containing AMMO as a precursor decreased from 131o to 119o after washing as five times. The highest contact angle values were obtained for F, H, FB and HB samples containing TDFOES and HDMS as precursors after washing. Figure 2 shows an image of the contact angle of FB sample containing TDFOES as a precursor.
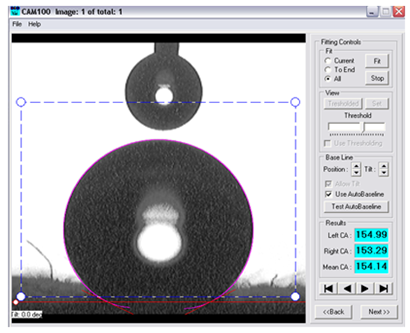 | Figure 2. Image of the contact angle of FB sample |
4.4. Tear Strength of the Fabrics
- The tear strength values (in the direction of the warp) of the fabric samples treated with nanosols by using the sol-gel process decreased as those of the untreated fabric samples. The tear strength values of the FB and HB samples prepared in basic conditions (6.67 N and 6.80 N, respectively) were higher than those of the F and H samples prepared in acidic conditions (5.84 N and 5.24 N, respectively). The tear strength values of the F and H samples were lower than those of the Conv. samples (7.36 N). When the tear strength values of the VTEO, FES, Zr, TIPT, AMMO and GLYE samples were investigated, the tear strength values of the VTEO samples (1.03 N) significantly decreased and the tear strength values of the FES, ZrA, TIPT, AMMO and GLYE samples changed between 4.16 N and 5.64 N. The tear strength values of the FES samples (5.64 N) were relatively high, while the tear strength values of the GLYE samples (4.16 N) were relatively low because of the cross-linking effect of the GLYEO precursor.
4.5. Whiteness Features of the Fabrics
- The whiteness values of the fabric samples treated with nanosols decreased compared with those of the untreated fabrics. The highest reduction of the whiteness values (from 81.11 to 61.65 as Stensby) of the fabric samples was observed for the AMMO samples. This could be due to the presence of nitrogen dioxide on the surface of the fabric samples. The whiteness values of the H samples (76.38 as Stensby) were higher than those of the other samples. The whiteness values of the HGL samples (75.68 as Stensby) containing HDMS and Guard AFB (5 g/l) did not significantly change like those of the H samples (76.38 as Stensby) treated in optimum conditions. The whiteness values of VTEO, FES, Zr, TIPT, AMMO and GLYE ranged between 61.65 for the AMMO samples and 74.38 for the Zr samples.
4.6. Bending Length of the Fabrics
- The bending length values of the fabric samples treated with nanosols in acidic conditions ranged from 4.90 cms to 2.00 cms, while the bending length values of the fabric samples treated with nanosols in basic conditions ranged from 1.90 cms to 1.70 cms. It was deduced that the fabric samples treated with nanosols in basic conditions were softer than the fabric samples treated with nanosols in acidic conditions. The bending length values of the Conv. samples (2.35 cms) did not significantly change like those of the untreated fabric (2.30 cms). The bending length values of the VTEO, FES, Zr, TIPT, AMMO and GLYE samples ranged between 2.00 cms for the FES samples and 4.20 cms for the VTEO samples. While the rigidity of the FES samples did not increase like that of the untreated fabric, the rigidity of the other fabric samples increased like that of the untreated fabric.
4.7. SEM Analysis
- Figure 3 presents SEM images of treated and untreated cotton fabric samples. The film layer interconnecting the fibres on the F, H, H, HG, HGL, ZrG and AMMO samples can be seen. It is clear from Figure 3 that the surface morphology of the VTEO, GLYE and Conv. samples was smoother than that of the untreated cotton fabric. It was deduced that thin film layers were formed on the surface of the treated fabrics. The thickness of the treated and untreated fibres was about 12 micrometres. In addition, some cracks on the film layer of the surface of the Zr samples were determined while the FES and TIPT samples had a particle kind of coating on their surface.
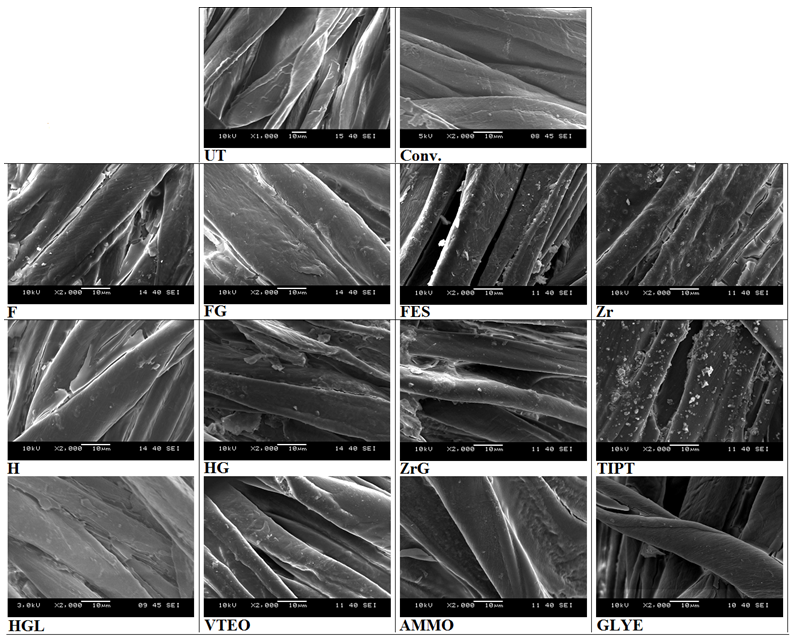 | Figure 3. SEM images of treated and untreated fabric samples |
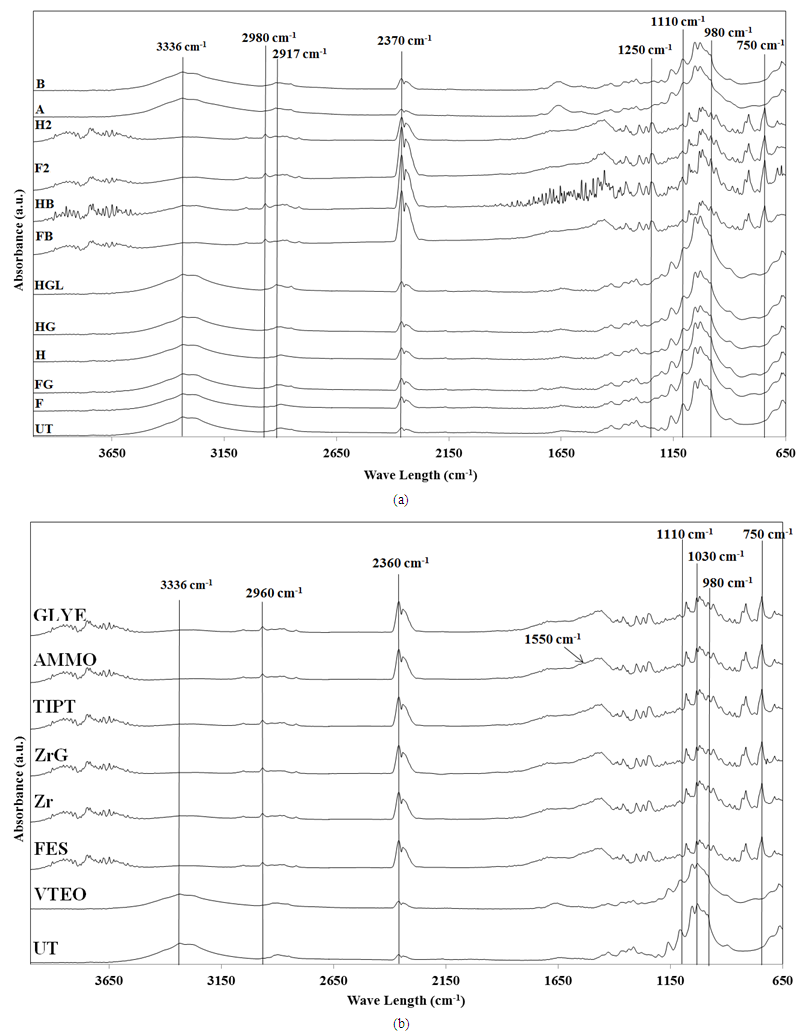
4.8. FTIR-ATR Analysis
- Figures 4a and 4b show the FTIR-ATR absorbance spectra of the untreated and treated fabric samples. It should be borne in mind that the bands between 3200 and 3600 cm-1 are due to O-H stretching and those at 2200–2600 cm-1 are due to C-H stretching frequencies. The presence of the CH2 and CH3 groups can be observed at the 2400–2900 cm-1 range corresponding to their vibration modes. The spectra of the F, FG, H, HG and HGL samples did not show a significant change compared with the spectra of the untreated fabric. The peaks for SiO2 were overlapped by the broad bands of cellulose. The increases at some peak intensities were detected for spectra of the other samples. The common features that appear in the wavelength band range of 1000 cm-1 to 1110 cm-1 correspond to the stretching vibrations of Si=O and also to the contributions of Si-O bonds [34]. The bands at 800 cm -1, 1200 cm-1, 950 cm-1, 980 cm-1 and 1100 cm-1 may be assigned to the vibration of Si6O18, Si2O6, Si2O5 and SiO2 bands for the fabric samples.
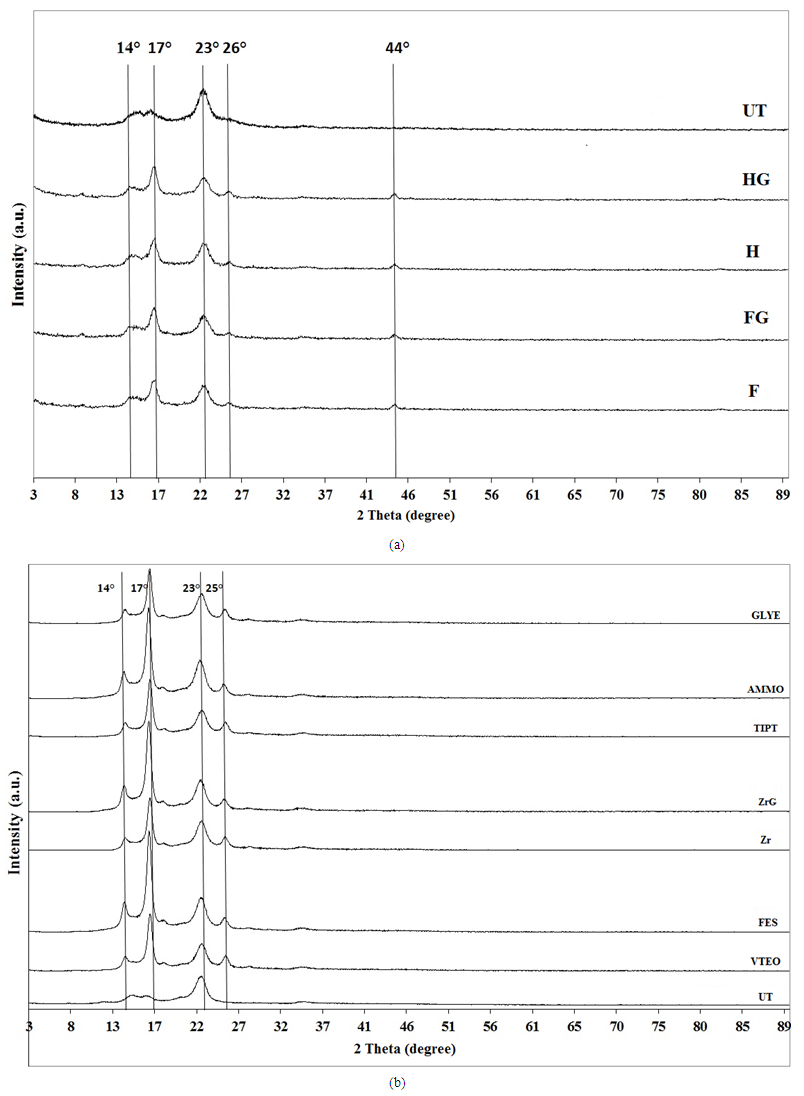
4.9. XRD Analysis
- Figures 5a and 5b present XRD patterns in the 2θ range 3–90° of the treated and untreated fabric samples. The SiO2 peaks for the fabric samples treated with nanosols can be found at 2θ–14.6°, 19.9°, 21.5°, 22.5°, 26.1°, 30°, 34°, 38°, 42.3°, 48° and 59.2°. The intensity of the peaks at 14°, 17° and 26° for the fabric samples treated with nanosols increased compared with the intensity of these peaks for the untreated fabric samples. For the F, FG, H, HG and HGL samples, a new peak occurred at 44°. In the literature, the characteristic peak of pure SiO2xerogel films was about 26° and has a wide and amorphous nature [34, 54]. No crystalline peak of SiO2 was observed because the films on the fabrics were prepared at low temperature.
5. Conclusions
- In summary, the development of a hydrophobic and oleophobic cotton fabric by using a sol-gel process was the aim of our study. In this respect, cotton fabric was treated with silane-, Zr- and Ti-based nanosols containing TEOS, TDFOES, HDMS, VTEO, ZrA, FES, TIPT and GLYEO as precursors with or without Guard AFB with different concentrations using a pad-dry-cure process. The effect of acidic and basic conditions, the concentration of precursor and adding Guard AFB with different concentrations on the water/oil-repellency properties of cotton fabric treated with silane-based nanosols containing TDFOES and HDMS as precursors was investigated. In this way, optimum conditions were established for the H samples. However, the H samples do not have oil-repellency properties. Thus two-thirds and one-third of the amount of concentration of Guard AFB as a conventional water/oil-repellent agent in a conventional recipe were added to this nanosol for the HG and HGL samples. It was deduced that the optimum conditions for producing a water- and oil-repellent cotton fabric was determined for the HGL samples containing a 4 ml concentration of HDMS and 5 g/l of Guard AFB in acidic conditions. In SEM images, it was observed that thin film layers on the surface of the treated fabrics were formed. It was concluded that a water/oil-repellent cotton fabric could be produced by using a sol-gel process, comparable with the conventional process.
ACKNOWLEDGEMENTS
- The study was supported by The Scientific and Technological Research Council of Turkey (project number 110R011) and Pamukkale University Scientific Research Projects Coordination Unit (project number 2010BSP012). The authors also greatly acknowledge Adnan Saka from Rudolf Duraner company’s support for supply conventional chemical agents, Assoc. Prof. Dr. Isil Birlik for support in instrumental analysis.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML