-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Textile Science
p-ISSN: 2325-0119 e-ISSN: 2325-0100
2015; 4(4): 78-83
doi:10.5923/j.textile.20150404.02
Influence of Auxiliaries in Dyeing of Wool with Acid Dyes
Sani Muhammad Gumel1, Abdulkadir Abdulmalik2, Shehu Habibu3, 4, Magaji Ladan1
1Department of pure and Industrial Chemistry, Bayero University, Kano, Nigeria
2Rabiu Musa Kwankwaso College of Advanced and Remedial Studies, Tudun Wada, Nigeria
3Department of Chemistry, Federal University, Dutse, Nigeria
4Department of Chemistry, Faculty of Science, University of Malaya, Kuala Lumpur, Malaysia
Correspondence to: Shehu Habibu, Department of Chemistry, Federal University, Dutse, Nigeria.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
The influence of ionic auxiliaries on absorption of the Acid milling CI Acid Blue 80 (Sandolan Milling Blue N-BL 150) dye on wool fibre and on the colour changes of the dyed fabric has been investigated. The motivation behind this work is possible reduction in the dyeing temperature of conventional dyeing. The absorption and the colour characteristics of dyed fabric, including light and washing fastness were examined. An anionic and cationic auxiliaries based on Lissapol D (ICI), the sodium salt of cetyl-oleylsulphate and dispersol (CWL), an ethylene oxide-amine condensate respectively, enable dyeing at low temperature. Colour characteristics and improvement in light and wash fastness of the dyed fabrics was achieved. The variation of hue and levelness due to the use of these auxiliaries or the difference in dyeing temperatures is reported.
Keywords: Acid dyes, Auxiliaries, Exhaustion, Wool
Cite this paper: Sani Muhammad Gumel, Abdulkadir Abdulmalik, Shehu Habibu, Magaji Ladan, Influence of Auxiliaries in Dyeing of Wool with Acid Dyes, International Journal of Textile Science, Vol. 4 No. 4, 2015, pp. 78-83. doi: 10.5923/j.textile.20150404.02.
Article Outline
1. Introduction
- Low temperature dyeing of wool has been of much interest for the most recent two decades in light of the extensive measure of advantages in the quality of the products as compered with the wool dyed by conventional method. The advantages include less yellowing, higher fabric abrasion resistance, and elongation at break of yarns, improve in the dye bath exhaustion as well fastness properties [1, 2]. In contrast, relative high temperature of the conventional dyeing process can weaken the fibre structure. Consequently, imparts unfavorable chemical and mechanical properties on the fabrics.However, low temperature dyeing affects the surface of textile materials physically and chemically without altering their bulk properties. Moreover, milling acid dyes do not penetrate wool fibres at low temperatures resulting in an unlevelled dyeing which is a major problem [3]. There have been many reports concerning the modification of the surface of wool and other textile materials using various methods, aimed at improving the adsorption [4, 5], spinnability, hydrophilicity [6, 7], depth of shade [8], shrink-resistance [9], oil repellency, fastness properties [10-12] and levelness. Pretreatment of wool with polar organic solvents, enzymes [13-16] alkali treatments [17] or certain anionic and nonionic surfactants [18] is the basis for new ways to deal with colouring of wool fabrics. The use of auxiliaries allow for wool to be dyed under mild conditions at 80-95°C with acid and pre-metallized dyes [19]. Studies have shown that the presence of these auxiliaries not only increase the degree at which dyes are taken up by fibre, but also improves levelness [20]. It has also been reported that low-temperature dyeing can reduce the cost of electricity in the dyeing process by about 20% as well as reducing the greenhouse emissions and environmental pollution [13, 21, 22]. Conventionally, the application of acid dyes did not require the use of auxiliaries as it could be controlled by the use of high initial pH and low temperature and by the modification of these variables as dyeing preceded. However, this conventional technique, although basically sound, presented certain problems in practice. For example, rapid rise or inconsistent pH changes at high temperature could basically annul the effect of the care exercised in the earlier stages of dyeing and little could be done to overcome uneven or unwanted dyed product [23]. In this study, wool fabrics were treated with Lissapol D (ICI), anionic agent), and Dispersol CWL (ICI), (cationic agent), at 60°C and at 97°C. The study has been concerned mainly with the application of milling acid dyes, rather than equalizing acid dyes, since the problems of obtaining uniformity are greatest with the milling acid dyes. The work has involved exploration of the three possible roles of the auxiliaries these are, exhaustion levelness, and colourfasness properties of the treated wool fabrics.
2. Materials and Methods
- The wool fabric used was treated according to ISO/F: 1985(E) and the dye used in this study was C.I Acid Blue 80 (Sandolan Milling Blue N-BL 150). The dye was purified by recrystallization three times, dissolving in hot dimethyl formamide, precipitated by acetone and then filtered. The dyeing auxiliaries Dispersol, Lissapol D, electrolyte and other reagents of analytical grade were used without further purifications.
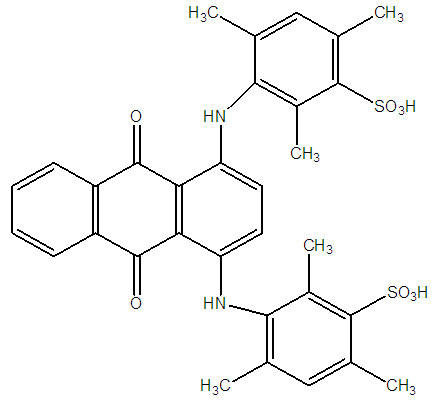 | Figure 1. Chemical Structure of (C. I. Blue Acid 80) Dye used |
2.1. Dyeing
- Dyeing were carried out at two different temperatures (60°C and 97°C), the dye bath containing the dye, 5.0% sodium dihydrogen phosphate (dehydrated), 1.5% disodium hydrogen phosphate to give pH of 6.5 at goods to liquor ratio of 1:30 when treated 2% Lissapol D or Dispersol CWL and 10% NaCl. Dyeing was also carried out in the absence of auxiliary. Dye concentration in the dye bath was measured at the start, after 10, 20, 30, 40 and 50 minutes, to determine the exhaustion [3, 23, 24]. The absorbance were measured spectrometrically using Spectrum lab 7525 from 25% pyridine-water solution at a wavelength of 590 nm λ max to determine the concentration of the dye. The percentage of dye bath exhaustion was calculated according to equation (1) below [3]:
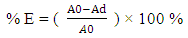 | (1) |
2.2. Evaluation of Colour Fastness Properties
2.2.1. Fastness to Light
- To Artificial light MK1 fitted with mercury-tungsten (MBTF) 500 watt lamp. The samples were exposed together with blue wool standards for about 96hours and assessed. Light fastness of the dyed samples were assessed using standard methods (B.S. 1006). The samples were exposed to artificial MK1 fitted with mercury-tungsten (MBTF) 500 watt lamp. The samples were exposed together with blue wool standards for about 96hours and assed [25].
2.2.2. Fastness to Washing
- Fastness to washing of dyed wool fabric was assessed using standard method (ISO 105-CO2 (1989), [26]. The fastness to washing were carried out by cutting the specimens to 5cm by 5cm and placed in between two pieces of undyed white materials of the same size, the samples were sewn together and made in to a composite specimen. The composite specimens were separately immersed in to washing liquor containing 100cm3 of 4gdm-3 detergent solution and agitated for 30 minutes at 50°C, the specimens were rinsed thoroughly, opened and air-dried. The change in colour of the dyed material and the degree of staining of the undyed fabric were assessed using grey scale.
2.2.3. Fastness to Rubbing
- The wash-fastness properties of the dyed samples were measured according to ISO 105-C01 standard. The color hue changes and the degree of staining on the adjacent yarns were measured after drying [26].
3. Results and Discussion
3.1. Exhaustion Curves
- The effect of auxiliaries is shown by the exhaustion curves in figure 2 and 3 below:
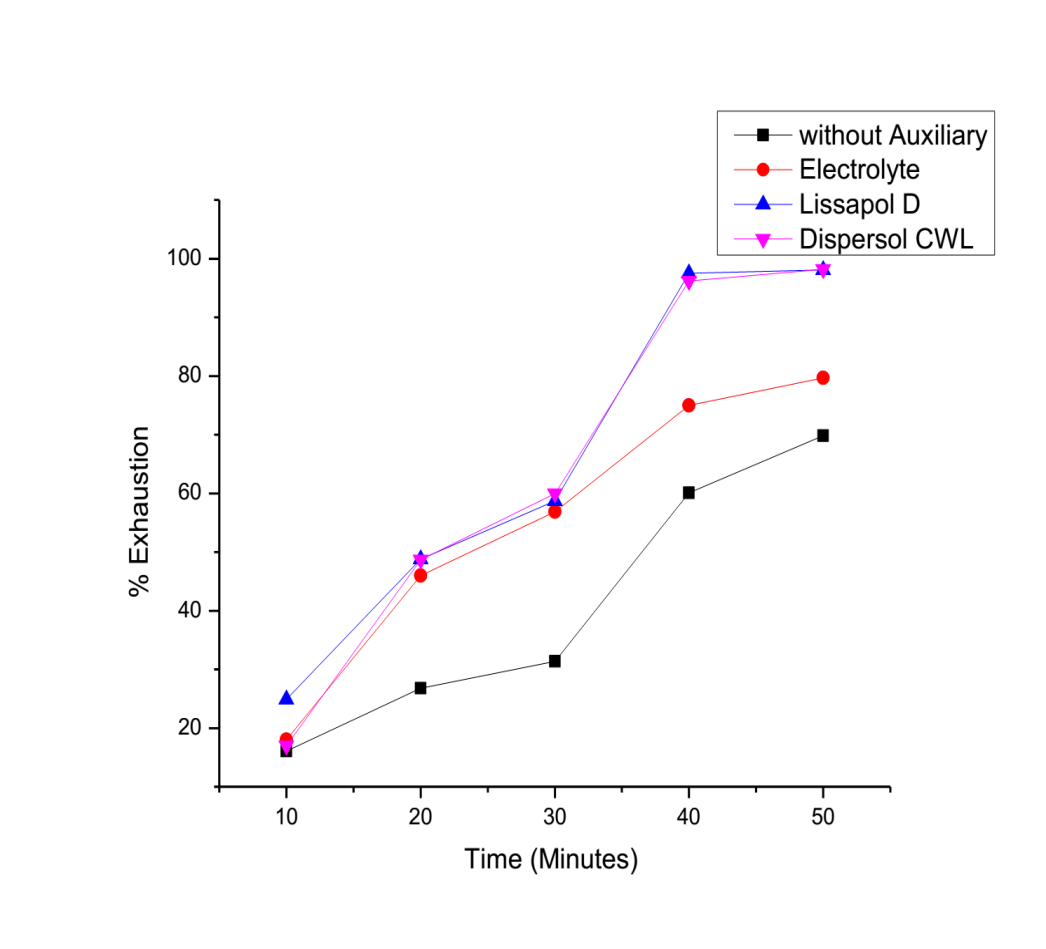 | Figure 2. Percentage Exhaustion against time of dyeing at 60°C |
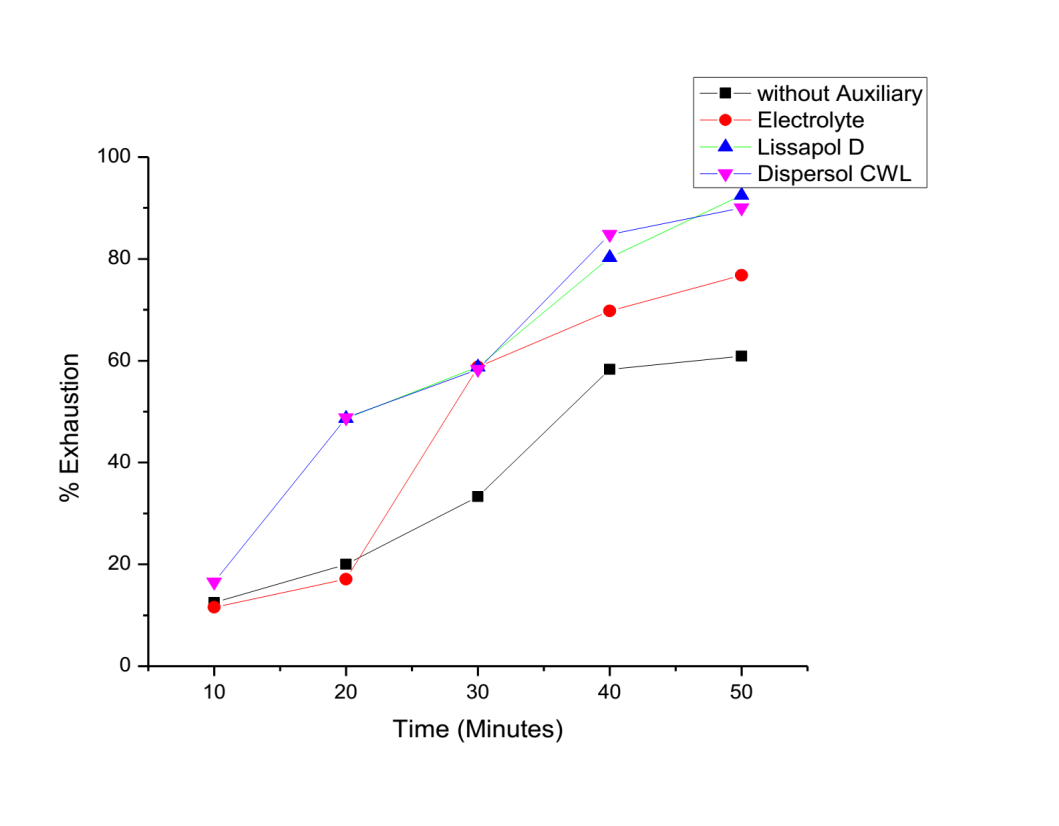 | Figure 3. Percentage Exhaustion against time of dyeing at 97°C |
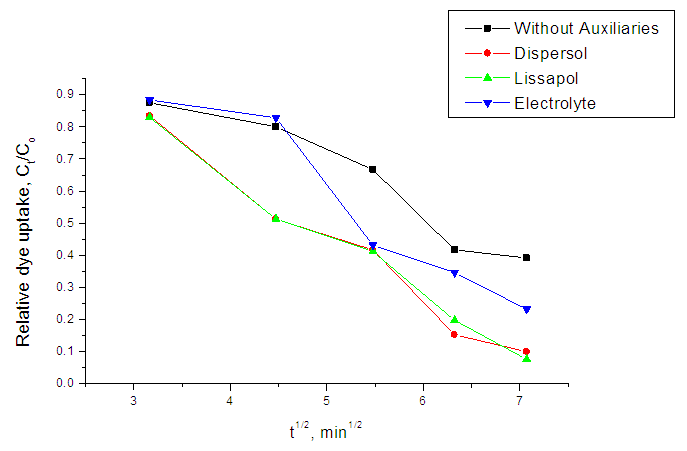 | Figure 4. Relative dye uptake versus dyeing time (t1/2) at 60°C |
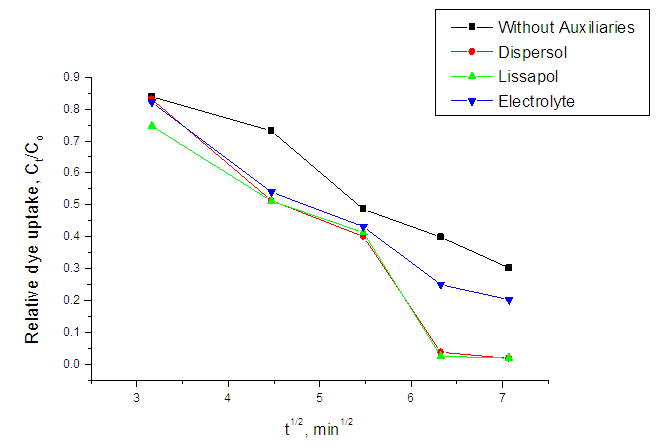 | Figure 5. Relative dye uptake versus dyeing time (t1/2) at 97°C |
3.2. Colour Fastness Properties
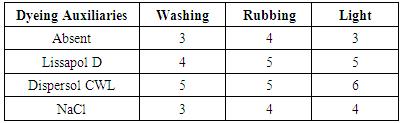
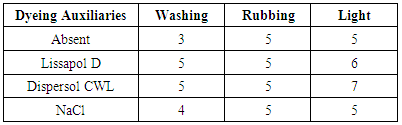
- The colour fastness properties of the dyed wool fabrics shows a resistance of a dyed fabric to external agencies such as light, washing and rubbing. wash fastness measure the resistance to laundry treatment while light fastness measure resistant to sun light [30, 31]. The colour fastness results of the dyed fabric is presented in table 1 and 2 below: 60°C and 97°C were summarized in table 1 and 2 below.From table 1 and 2 it can be observed that, the cationic Dispersol CWL show higher fastness properties at both temperatures probably, due to the formation of complex with the wool fabric. The wash fastness of the dyed wool fabric varied from moderate to very good when different auxiliaries were used and is poor in the absence of dyeing auxiliaries even at 97°C, the rubbing fastness of dyed wool fabrics is very good to excellent in dyeing using auxiliaries and fair in the absence of dyeing auxiliaries. The light fastness of the dyed wool fabric varied from moderate to good. The fastness properties of the dyed wool fabric in this research are comparable with those reported by [9, 32, 33].
|
|
4. Conclusions
- The dyeing behavior of wool at 60°C and 97°C has been studied using milling acid dyes and cationic (Dispersol CWL), anionic (Lissapol D) and an Electrolyte (NaCl) auxiliaries. It has been found that the presence of these auxiliaries aid in the diffusion of the milling acid dye in to the wool fibre. Dyeing rate as well as fastness properties were improved on application of these auxiliaries.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML