-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Textile Science
p-ISSN: 2325-0119 e-ISSN: 2325-0100
2015; 4(3): 53-59
doi:10.5923/j.textile.20150403.01
A Study on Phase Change Material with Reference to Thermal Energy Storage by Using Polyethyleneglycol-1000 to Create Thermo-Regulating Fabric
Asfandyar Khan1, 2, Md. Nahid Pervez1, 3
1Wuhan Textile University, Wuhan, China
2National Textile University, Pakistan
3Southeast University, Bangladesh
Correspondence to: Asfandyar Khan, Wuhan Textile University, Wuhan, China.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Over the world thousands of people are facing the thermal discomfort feels problem of textile fabrics. In this research we introduce phase change materials (PCM) that resolve the problem by creating thermo-regulating fabric with the help of increasing thermal energy storage. Polyethyleneglycol-1000 has been used as a PCM material. For conducting this experiment we produce microcapsules by using 2.5% PEG-1000with Na-alginate and making binder paste. After making two materials all are applied on fabric by rubber squeegee. PEG microcapsules are verified by using optical microscopy, FT-IR analysis and DSC studies. By measuring DSC result we got highest thermal energy storage of 2.5% PEG coated fabric than binder coated fabric that is 2842.5120 J/g and FT-IR analysis of this exhibited the peaks at 3400-2400cm−1, 3400-3300cm−1, and 1300-1100cm−1 these are the characteristic absorption peaks of -OH, -C-O-C and –OH stretching vibrations. On the basis of results we confirmed that fabric coated with 2.5% PEG microcapsules showed higher thermal energy storage capacity, than the binder coated fabric that enhance the thermo-regulating property of fabric.
Keywords: Phase Change Material (PCM), Thermal Energy Storage (TES), Micro-Encapsulation, Na-alginate, FT-IR
Cite this paper: Asfandyar Khan, Md. Nahid Pervez, A Study on Phase Change Material with Reference to Thermal Energy Storage by Using Polyethyleneglycol-1000 to Create Thermo-Regulating Fabric, International Journal of Textile Science, Vol. 4 No. 3, 2015, pp. 53-59. doi: 10.5923/j.textile.20150403.01.
Article Outline
1. Introduction
- In recent years, significant technical progress in textiles has been observed with the increasing of fundamental science application. The process concept of phase change is driven by solid-liquid and acclimatising properties of fiber which is attributing more tending by using phase change material (PCM) [1]. For making PCM process microcapsules are must be comprise with the help of polymerization procedure [2]. Enhancing the thermal behavior of textile fibers in 1987 scientists discovered a new pattern of technology which is integrating microencapsulated PCM [3]. The development of textiles intended to improve the thermal comfort of its user is closely connected with the trend of smart textile. For making longer period of time human organism According to the WHO temperature should be kept in (37 ± 1) °C [4]. Three types of heat storage were reported: sensible, latent, and chemical reaction. The storage of sensible heat is based on increasing the temperature of any substance without changing its phase. The storage of heat via chemical reactions is based on the thermo physics of the reactions. The storage of latent heat is the most important type of heat storage [5]. PCMs are used to achieve latent heat storage. The storage of latent heat or thermal regulation is based on the transition of a material (PCM) between phases. When a phase change material is heated to its melting point, it absorbs heat during the change in phase from a solid to a liquid state. Phase change materials possess the ability to change their state with a particular grade of temperature. During phase change materials should be absorb energy other than energy smoothly going to the environment in the phase change stage when a reverse cooling process occurred [6]. PCM reacts directly to the environmental temperature changes and different areas body temperature. Microcapsules of PCM react with absorbing heat and store energy in liquefied PCM this phenomena occurs when temperature increase and immediately microcapsules get out storage energy when temperature decline and that time PCM solidify occurred [7]. Phase change materials are able theoretically to change state at nearly constant temperature and therefore to store large quantity of energy [8]. We can use PCM in active and passive heating-cooling system [9]. For bridging time gap between energy requirements and use thermal energy storage (TES) should kept storage some high or low temperature energy for after use [10]. For selection TES system in a particular application some factors is concern like Economics, storage capacity and process time duration, heat losses and going to be an essential method for TES utilization [11]. In textile field it is the most effective ideas of using Thermal energy storage of PCM with 15 to 35C melting point. Inadequacy of water PCM are more than 500 natural and synthetic [12]. PEG-1000 (Mw) was encapsulated using an in-situ polymerization technique. In 1980 century NASA was discovered Microencapsulation technology for overcome space suits problem. The properties of microcapsules are walls less than 1 µm thick and 20 - 40 µm in diameter with 80% - 85% of PCM. The small capsule size provides a relatively large surface area for heat transfer. The rate at which the PCM reacts to an external temperature change is very rapid [13]. In order to preventing drip off clothing melt situation PCM must be place into microcapsules. It is the process of enveloping microscopic sized droplets or particles in a shell material for the purposes of protection or controlled release, because PCM containing microcapsules must be durable and safe through the finishing process [14]. Some properties have to include in good PCM such as high thermal conductive, specific heat, fusion heat solid to liquid reversible, low vapor pressure and volume change when transition happened [15].In this article we use 2.5% PEG with sodium alginate to produce microcapsules. From the beginning of this process macro capsules were formed and crystals formed and finally microcapsules were formed. After making microcapsules we observed thermal energy of storage amount of coated fabric that is accelerate the creating thermo-regulating fabric.
2. Materials and Methods
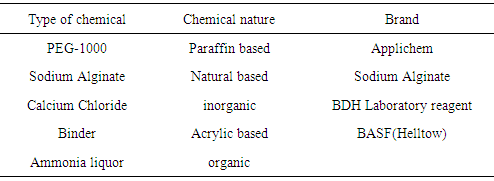
2.1. Materials
2.2. Chemical
2.3. Analytical Methods
- A DSC-60/60A Differential Scanning Calorimeter was used to study the thermal behavior of the microcapsules. The sample weight used for the testing ranged from 8-12 milligram. After the sample is ready, it is placed into the DSC cell and machine is turned ON. The experiment is designed onto the computer in which parameters like temperature range, temperature change interval, weights of sample, type of test, and comments are entered. Once the test is completely done, the DSC graph is opened in the DSC analysis software where we can integrate the peaks the enthalpy given out or absorbed during these processes. FTIR is a technique which is used to obtain an infrared spectrum of absorption, emission, photoconductivity, or Raman scattering of a solid, liquid, or gas. A Bruker FTIR study was important in our study to determine the presence of PEG in the microcapsules. The FT-IR spectrum is taken and the peaks are marked out with the help of a peak-finding tool to get the wavelength of all the peaks shown on the spectrograph. A stereo microscope of CzM6 model was used to determine the shape and size of the microcapsules.
3. Experimental
3.1. Encapsulation Procedure for PEG 1000
- Recipe for microencapsulation1. Polyethylene Glycol = 2.5% 2. Sodium Alginate = 1.0% 3. Calcium chloride = 2.0%
3.2. Formation of Macro Capsule
- First of all, a mixture of polyethylene glycol (PEG 1000) and hot water was prepared in 250.0 ml glass beaker in which 2.5g of PEG was added to 100.0 ml water, followed by addition of 1.0g of sodium alginate. The mixture was thoroughly stirred with the help of an electric stirrer for 10 minutes. Then a mixture of calcium chloride and hot water was prepared in 250.0 ml glass beaker in which 2.0 g of calcium chloride was added to 100.0 ml water. The solution containing sodium alginate and PEG 1000 was added drop wise to solution containing calcium chloride by using syringe. As a result, macro capsules were formed instead of micro capsules.
3.3. Formation of Crystals Instead of Microcapsule
- Again a mixture of polyethylene glycol (PEG1000) and hot water was prepared in 250.0 ml glass beaker in which 2.5g of PEG was added to 100.0 ml water, followed by addition of 1g of sodium alginate. The mixture was thoroughly stirred with the help of an electric stirrer for 10 minutes. Then a mixture of calcium chloride and hot water was prepared in 250.0 ml glass beaker in which 2.0 g of calcium chloride was added to 100.0 ml water. This time the solution containing calcium chloride was added drop wise to solution containing sodium alginate and PEG 1000 by using syringe with high speed stirring. The added droplets were retained in calcium chloride solution for 15 minutes to complete the curing reaction and to produce microcapsules. The solution was filtered by using filter paper and washed with water to remove the remaining calcium chloride, dried in oven and examined with microscope but in result crystal like shapes were formed.
3.4. Formation of Microcapsule with 2.5% Concentration of PCM
- A mixture of polyethylene glycol (PEG-1000) and hot water was prepared in 250.0 ml glass beaker in which 2.5g of PEG was added to 100.0 ml water, followed by addition of 1.0g of sodium alginate. We added 5.0g dispersing agent to the solution. The mixture was thoroughly stirred with the help of an electric stirrer for 10 minutes. Then a mixture of calcium chloride and hot water was prepared in 250.0 ml glass beaker in which 2.0 g of calcium chloride was added to 100.0 ml water. The solution containing calcium chloride was added drop wise to solution containing sodium alginate and PEG-1000 by using syringe with high speed stirring. The added droplets were retained in calcium chloride solution for 15 minutes to complete the curing reaction and to produce microcapsules. The solution was filtered by using filter paper and washed with water to remove the remaining calcium chloride. The precipitate was removed from filter paper and put in to a conical flask; dispersion was made by using magnetic stirrer by adding water to the precipitate present in conical flask. The dispersion made was examined with microscope; it was proved that microcapsules were formed.
3.5. Application Method on Fabric
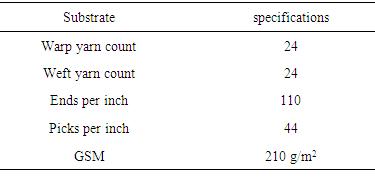
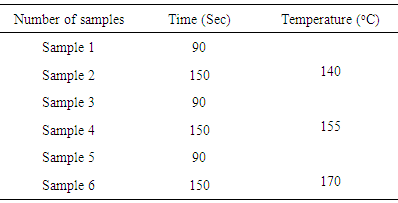
- Stock paste recipe:1. Thickener = 40 g/kg2. Ammonia liquor = 10 g/kg3. Binder (Urethane based) = 50 g/kgThe stock paste was prepared according to above recipe and microcapsules were also prepared. Then the stock paste and microcapsules were mixed with ratio of 50:50 by using stirring rod. The paste was formed and then applied on to the fabric with the help of rubber squeegee. The angle of rubber squeegee was kept at 75C. The paste was applied in the form of two layers. After the application, the six samples were dried at a temperature of 110C. (Table 3)
|
4. Result and Discussion
4.1. FT-IR Analysis
- It is clear that FT-IR spectra of standard cotton sample (100%) is shown in Figure 1 (a) functional group of sodium alginate is carboxylic acid which two spectra showed different peaks at 3400-2400 cm-1, and 1400-1440 cm-1, and near ketones spectra is 1300-1101cm-1. These peaks are attributed to O-H, -C-O stretching vibrations and -OH bending. Again FT-IR spectra of coated fabric with binder is shown in (b) functional group of sodium alginate is carboxylic acid which spectra showed peaks at 3400-2400 cm-1, Then for Aldehydes peaks at 2850-2750 cm-1, and for Anhydrides spectra is 1300-900cm-1. These peaks are attributed to O-H, -C-O and C-H aldehyde stretching vibrations here no bending vibrations occurred. For getting more comfortable behavior use PEG-1000 then observed FT-IR spectra of coated fabric with 2.5% PEG is shown in (c) The resulting FT-IR spectra of both samples, PEG encapsulated microcapsules show the expected peaks at 800-1200 cm-1, and around 2400-3400cm-1 show the presence of alcohols and ethers functional groups of polyethylene glycol and carboxylic acids functional group of sodium alginate. These peaks are attributed to O-H, -C-O-C (dialkyl) and O-H bond stretching vibrations here no bending vibrations occurred.
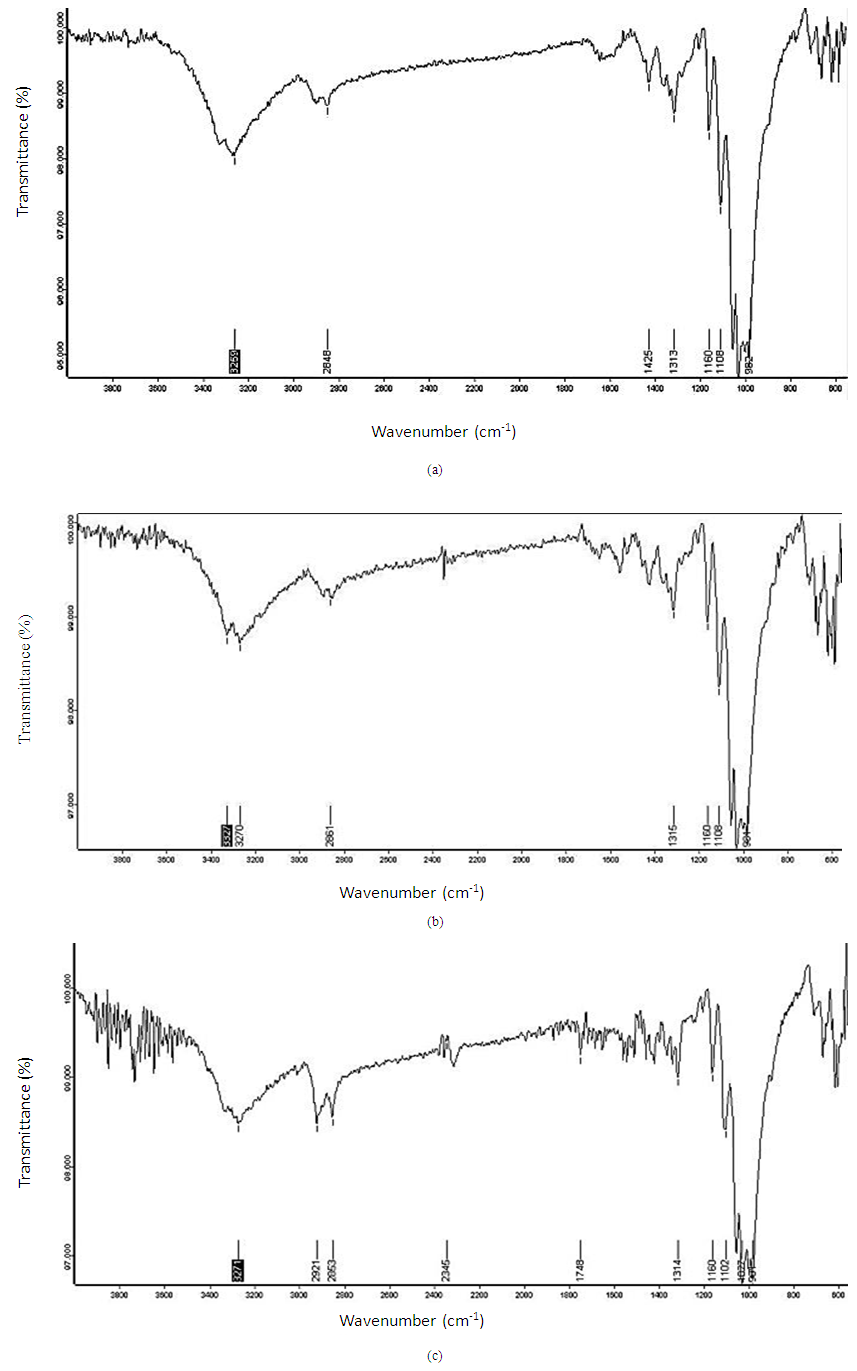 | Figure 1. FT-IR spectra of (a) 100% Cotton (b) cotton with binder and (c) cotton with 2.5%PEG |
4.2. DSC Analysis
- The DSC results are obtained and shown in Figure 2 (a) the heat analysis was done of the standard sample to examine its thermal behavior. The sample was heated up to 220C at a constant rate of 10C /min. As shown negligible heat is absorbed by the standard cotton sample which may be due to the amount of moisture present in the concerned sample. It is concluded that our standard sample shows an exothermic behavior. (b) It showed that the heat storage capacity of the coated fabric with binder was 1557.8 J/g in the range from 40C to 130C which shows that cotton coated fabric shows an endothermic behavior in the specified range of temperature. In case of (c) The heat analysis was done of the 2.5% PEG microcapsules coated fabric to examine the thermal behavior of PEG. The sample was heated up to 140C at a constant rate of 10C /min. The phase change temperature Tm of the microcapsules was found to be around 92.8C. The heat storage capacity of the microcapsule was 2842.5120 J/g. When the PCM microcapsules are heated, they absorb energy and go from a solid state to a liquid state. This phase change produces a temporary cooling effect in the clothing layer. If the PCM microcapsules are cooled down below the freezing point of PCM material, the material will change back to solid state from the liquid state, releasing heat and thus developing a temporary warming effect. Near 30C the recrystallization of the PCM material can be seen causing the exothermic reaction, thus releasing heat to the fabric.
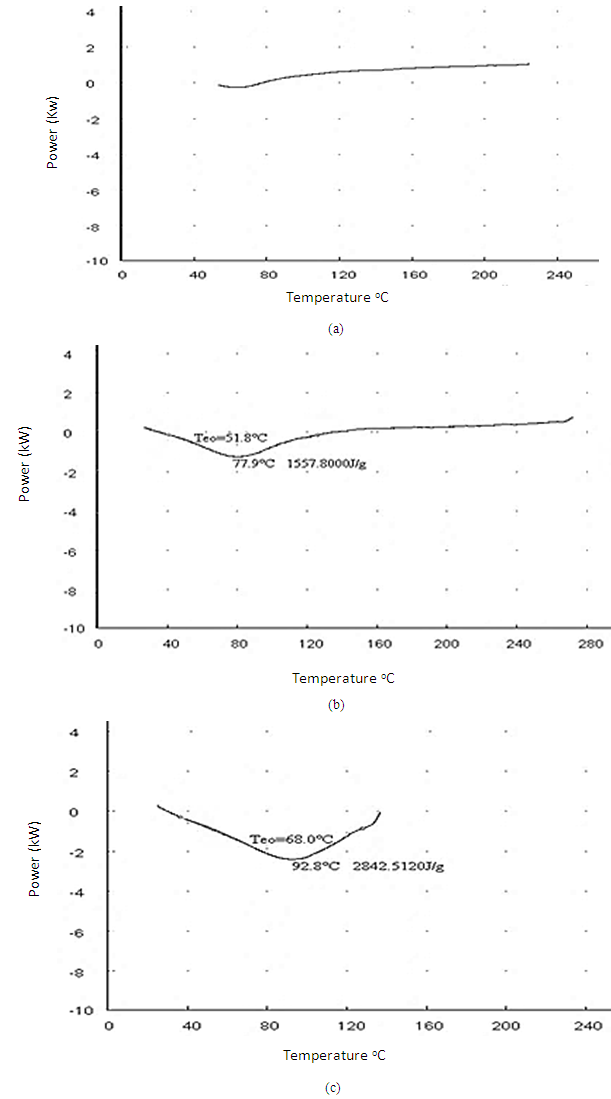 | Figure 2. DSC analysis of (a) 100% Cotton (b) cotton with binder and (c) cotton with 2.5%PEG |
4.3. Optical Microscopy Analysis
- For analysis optical microscopy of the microcapsules we used the stereo microscope of CzM6 model with magnification of 180x.the images taken by the camera are shown in Figure 3 (a) the formation of macro capsules instead of microcapsules then (b) show that crystals were formed rather than microcapsules because of aggregation among capsules and (c) show that confirmation of microcapsules.
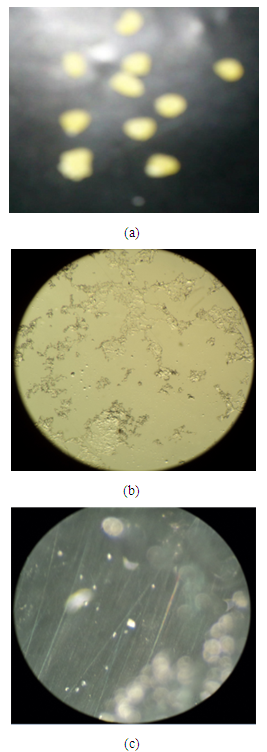 | Figure 3. Optical Microscopy (a) Macro capsules (b) Formation of crystals instead of microcapsules and (c) Confirmation of microcapsule |
|
5. Conclusions
- On the basis of the results obtained from the measurements observed that FTIR analysis showed that functional groups of PEG and sodium alginate go higher wave number cm-1. Furthermore, DSC analysis incorporated energy storage value of microcapsules with PEG was 2842.5120 J/g. The cooling effect of this PEG-1000 treated coated fabric provides to keep using wear in cool environment. Further work using different concentration of PEG with a higher molecular weight and microcapsule size distribution may prove to be useful. In today’s world of developing technologies, the technique of microencapsulation is applied in almost all the fields. It has become a prominently effective technique which enhances the property imparted to the fabric and assures its durability. Microcapsules performed their function effectively under both heating and cooling conditions, and endothermic and exothermic phase transitions took place within the intervals coinciding with those of PCM.Finally it can say that from the perspective of the human physiology-clothing-environment system and thermal physiology, it is challenging for textile and clothing designers to achieve effective cooling or warming function and reasonable duration using textiles incorporated with phase change materials but now a days Designers focus on fashion and appearance, whereas safety and protection engineers and physiologists emphasize functions in terms of developing functional and protective clothing by using Phase change materials (PCMs) because they are responsive to temperature change by absorbing or releasing heat, and thus have potential of self-regulating human skin temperature and thermal sensation.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML