-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Textile Science
p-ISSN: 2325-0119 e-ISSN: 2325-0100
2014; 3(4): 59-63
doi:10.5923/j.textile.20140304.01
Estimation of Total Hardness of Bath Water in Knit Dye Houses in Bangladesh and Study of Its Effects
Mohammad Gias Uddin1, Abu Sayeed Md. Atiquzzaman2
1Department of Textile Engineering, Faculty of Engineering, Ahsanullah University of Science and Technology, Dhaka, Bangladesh
2Department of Textile Engineering, Faculty of Engineering, Ahsanullah University of Science and Technology, Dhaka, Bangladesh.
Correspondence to: Mohammad Gias Uddin, Department of Textile Engineering, Faculty of Engineering, Ahsanullah University of Science and Technology, Dhaka, Bangladesh.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
It is well known that the hardness of process water can cause different types of problems in textile processing. But it is considered that not only the water, but also some chemicals like electrolyte and metal contamination of fiber by naturally or during production are responsible for hardness of bath water. That is why the total hardness of bath water should be maintained at a minimum level. In this work, emphasize has been given to determine the hardness of water, salt and substrates of knit dyeing industries in Bangladesh. Samples of water, salts, grey fabrics and sequestering agents from fifteen different knit dyeing industries situated in different industrial zones surrounding Dhaka in Bangladesh were collected and total hardness of bath water was determined. At the same time, the negative influences of water hardness of the bath in knit dyeing industries and the performance of water softening chemicals were also investigated.
Keywords: Total hardness, Process water, Bath water, Knit dyeing, Water treatment plant
Cite this paper: Mohammad Gias Uddin, Abu Sayeed Md. Atiquzzaman, Estimation of Total Hardness of Bath Water in Knit Dye Houses in Bangladesh and Study of Its Effects, International Journal of Textile Science, Vol. 3 No. 4, 2014, pp. 59-63. doi: 10.5923/j.textile.20140304.01.
Article Outline
1. Introduction
- In textile wet processing, the water quality plays a vital role in determining the product quality, consistency and production efficiency. The most important quality in governing the dye house quality is the water hardness. Consistent water quality plays a very significant role in the success of textile wet processing operations. During the time of textile processing, the hardness of water may vary due to metal content of some chemicals used in the processing, i.e. salts or electrolytes and also for the metal contamination in the textiles to be processed [1]. The textile industries in Bangladesh are sourcing their process water mainly from municipal water or deep tube well. Most of the textile dyeing industries is using Water Treatment Plant (WTP) to reduce the water hardness to a minimum level. They also use additional chemicals depending on the types of wet processing employed (i.e. pretreatment, dyeing, finishing, printing) to avoid the negative effects of residual hardness of water. Even though the industries are facing lots of problems for water hardness, this is due to the poor performance of water softening plant, sequestering agent and wrong calculation of hardness in bath water. Maximum industries in Bangladesh consider only the hardness comes from water, but they do not consider the negative influences of the chemicals and substrates that may contain the metal salts as impurities. That’s why the dyeing industries in Bangladesh still face lots of problems comes from water hardness even after having the WTP.
2. Effects of Using Hard Water in Textile Industry
- The use of hard water in knit processing industries can cause some serious consequences. These include:v If calcium and magnesium ions are not sequestered, there is the strong possibility of their combining with natural “soaps” which have been generated during the alkaline scouring process, to form waxy substances. These have been referred to as “lime soap deposits”. They can deposit not only on the substrate itself, but also on the surfaces of machinery [2]. It is not only the current production batch which is at risk, but also subsequent ones.v Re-deposition of dirt and insoluble soap on the fabric being washed- this can cause yellowing and lead to unlevel dyeing and a poor handle [3].v The presence of iron, copper or manganese can cause catalytic and uncontrolled decomposition of hydrogen peroxide. This can result in inefficient use of (expensive) peroxide, reduced degree of whiteness, loss of tensile strength of the fiber and “pinhole” damage [1].v Calcium and magnesium ions reduce the solubility of anionic dyes causing them to aggregate or even precipitate onto the fibers. They usually remain on the fiber surface as particulate deposits [2]. The net results are lower color yield; unlevel dyeing (ruined batches), spots and stains which are difficult to remove, change of shade, and even contaminated machines which can ruin subsequent dyeing.v Precipitation of some dyes as calcium or magnesium salts and hard water can, therefore, cause serious interference with the application of such dyes [3, 4].vWater hardness also reduces the activity of the enzymes used in textiles, affects the compatibility of chemicals used in textile finishing, forms scale on equipment, boilers and pipelines etc. [3].
3. Experimental Work
3.1. Specimen
- Raw water and process water (Soft), salt, sequestering agents and cotton single jersey knitted fabrics were used as specimen.
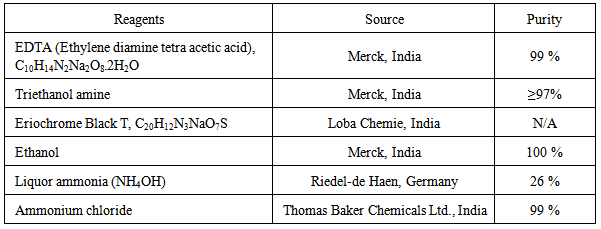
3.2. Reagents
3.3. Methods
3.3.1. Estimation Method of Total Hardness for Both Raw Water and Soft Water
- 1 ml of ammonia buffer solution (NH4OH + NH4Cl) [145 ml of liquor ammonia of specific gravity 0.88 + 15 gm NH4Cl in 105 ml of distilled water to make 250 ml solution to give a pH of 10] was added to a 50 ml of original water sample. 3-4 drops of Eriochrome Black T indicator (0.2 gm dye in 15 ml of triethanolamine and 5 ml ethanol) were also added. Then titration was carried out against 0.01 M EDTA solution until color changed from wine red to pure blue with no reddish tinge remaining [5]. If more than 20 ml of EDTA solution required, the test was repeated with a smaller sample made up to 100 ml with distilled water [6]. Then using (1), the total hardness was calculated in terms of ppm of CaCO3.
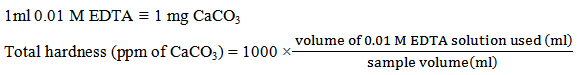 | (1) |
3.3.2. Estimation Method of Total Hardness of Salt
- Hardness was measured by analytical titration of a solution of salt in distilled water at a concentration of about 80 g/l [7]. 8 gm of salt was taken in 100 ml of distilled water in a beaker and made a clear solution by stirring. Then the solution was heated at 80°C for 10 minutes and cooled down. 50 ml of this solution was taken in a conical flask and titrated in the similar way by using (1) for estimation of total hardness in the salt.
3.3.3. Estimation Method of Total Hardness of Fabric
- Fiber hardness level can be determined using simple extraction technique.2 gm fabric sample was taken and cut into small pieces in the 100 ml of distilled water into a beaker. After heating at 95°C for 30 minutes, the solution was cooled down [7]. Then 50 ml water was taken from the beaker into a conical flask and titrated in the similar way by using (1) for estimation of total hardness in the fabric.
3.3.4. Estimation Method of Total Hardness of Blank Dyebath with Sequestering Agent
- Blank dyebaths were prepared, using 2 gm of fabric (Small cut pieces), 8 gm of salt and 0.1 gm of sequestering agent (1g/l) in 100 ml of soft water in a beaker. After heating at 95°C for 30 minutes and cooling down, 50 ml water was taken from the beaker without any fabric piece into a conical flask and titrated in the similar way by using (1) for total hardness of the bath.
4. Results and Discussion
4.1. Total Hardness of Raw and Soft Water
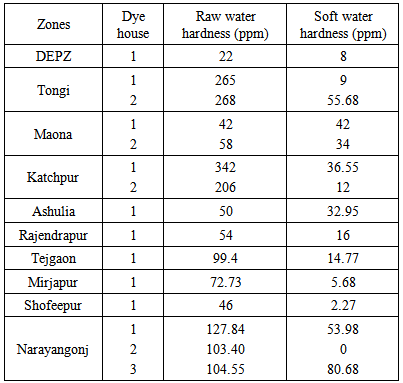
- Total hardness of raw and softened water of various dyehouses in Bangladesh are shown in Table 2. Tongi, Katchpur and Narayangonj are the three riverside zones in Bangladesh. The hardness found in raw water at the dye houses of these three zones were more than the other dyehouses. This might be due to the discharge drained in the river from the limestone industries of the riverside zones. In dye house 1 of Maona zone, no water softening plant was used and they used raw water directly in dye bath though it is not accepted to use raw water directly in a dye house. The other dye houses used water softening plant to reduce water hardness satisfactorily, but it was found that the hardness in softened water at the dye houses 1 and 3 of Narayangonj zone and dye house 2 in Tongi zone were more than the others. The best result was found in the dye house 2 of Narayangonj zone. Generally, the acceptable total hardness content for process water is within 0-25 ppm [8], total hardness found in the process water of the dye houses of DEPZ, Rajendrapur, Tejgaon, Mirjapur, Shofeepur, dye house 1 of Tongi and dye house 2 of Katchpur has also fallen within the range.
|
4.2. Salt Quality
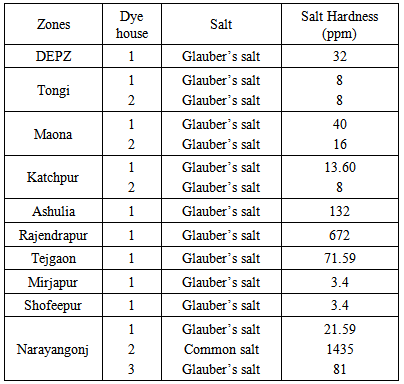
- Total hardness of salt in different zones is shown in Table 3.
|
4.3. Substrate Quality
- The total hardness of cotton single jersey knit fabric is shown in Table 4.
|
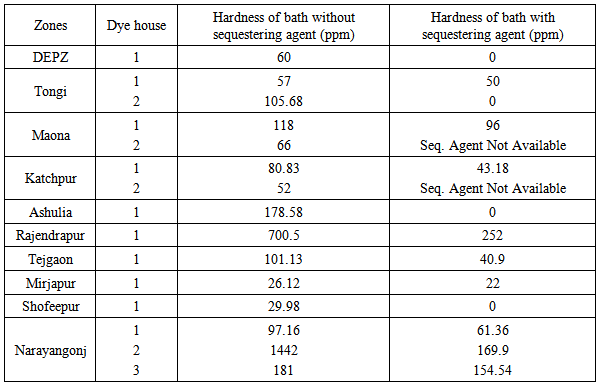
4.4. Total Hardness of Bath Water
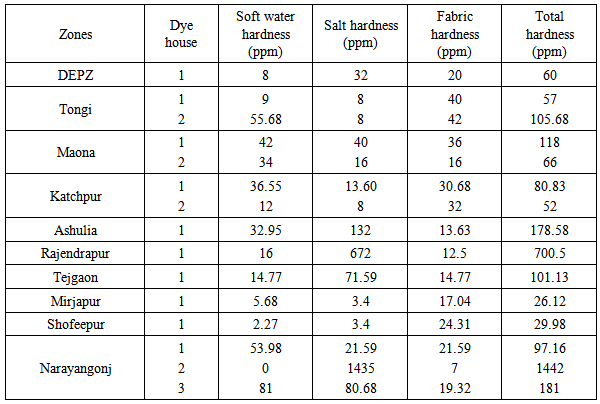
- Total hardness in bath water of the different dye houses are summarized in Table 5. From the overall experiment, the total hardness of the bath water at the dye houses of Mirjapur and Shofeepur was found quite satisfactory, because these two dye houses maintained soft water quality, salt quality and fabric quality properly. Results were also achieved ranging from 50 to 70 ppm in the dye house of DEPZ, dye house 1 of Tongi, dye house 2 of Maona and dye house 2 of Katchpur. The total hardness was estimated 1442 for dye house 2 of Narayangonj and 700.5 for Rajendrapur dye house due to the excessive salt hardness; also salt hardness increased the total hardness of the dye houses of Ashulia, Tejgaon and dye house 3 of Narayangonj.
|
4.5. Quality of Sequestering Agent
- The impact of sequestering agent in blank dyebath is shown in Table 6.
|
5. Suggested Remedies
- v It is suggested to use an effective WTP and its proper maintenance and control are also very important. vThe use of effective water softening chemicals is also important for problem-free textile wet processing.vTotal hardness of the bath should be considered to avoid the problems caused by the metal ions and their salts.v Purity of salts should be confirmed.v Metal contamination of the substrate can be solved by demineralization.v Strength of sequestering agent should be determined. v Ensuring how much iron is removed from bath water by sequestering agent is also important apart from removing total hardness.
6. Conclusions
- Water quality is the first thing for successful wet processing in textiles. But the water quality might be varying during the textile wet processing. In the bath, water hardness is incorporated with the hardness coming from chemicals, substrates and other sources, thus making the total hardness of the bath. So it is necessary to identify all the potential sources of hardness and to take necessary steps to deactivate the negative effects of bath hardness. The total hardness of raw water was found more in the dye houses of riverside zones and these dye houses near the riverside zones in Bangladesh should strictly control the hardness of process water. Thus the consistent quality of the production in dye houses of Bangladesh can be ensured.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML