-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Textile Science
p-ISSN: 2325-0119 e-ISSN: 2325-0100
2014; 3(3): 39-43
doi:10.5923/j.textile.20140303.01
Production of Tissue Engineering Scaffolds from Poly Caprolactone (PCL) and Its Microscopic Analysis
Md. Mahabub Hasan1, Khandakar Abu Nayem1, Mohammad Billal Hossain1, Sharmin Nahar2
1Department of Textile Engineering, Primeasia University, Dhaka, 1213, Bangladesh
2Department of Textile Engineering, Atish Dipankar University of Science & Technology, Dhaka, 1213, Bangladesh
Correspondence to: Md. Mahabub Hasan, Department of Textile Engineering, Primeasia University, Dhaka, 1213, Bangladesh.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Electrospinning technique have been attracted growing attention since last few decades for producing tissue engineering scaffolds. Scaffolds produced from nanofibers for tissue engineering application is a large field. It is possible to produce continuous fibers from different types of polymer at the nanometer scale by electrospinning technique. The aim of the research is to produce tissue engineering scaffolds from Poly caprolactone (PCL) polymer solution by electrospinning technique and analyze its microscopic appearance to determine the morphology of nanofibers (scaffold). The effect of ethanol on fiber morphology is also observed, as the morphology of fiber is supportive for cell adhesion. It has been observed that the maximum number of fibers have been produced with the diameter of 200 to 300 nm and the produced scaffolds are not significantly affected by ethanol.
Keywords: Electrospinning, Nanofiber, Polycaprolactone, Scaffold
Cite this paper: Md. Mahabub Hasan, Khandakar Abu Nayem, Mohammad Billal Hossain, Sharmin Nahar, Production of Tissue Engineering Scaffolds from Poly Caprolactone (PCL) and Its Microscopic Analysis, International Journal of Textile Science, Vol. 3 No. 3, 2014, pp. 39-43. doi: 10.5923/j.textile.20140303.01.
Article Outline
1. Introduction
- In the early 1990s several research groups (notably that of Reneker and Rutledge who popularized the name electrospinning) demonstrated that many organic polymers could be electrospun into nanofibers. Nanofiber is defined as ‘having a diameter of less than one micron, although the National Science Foundation (NSF) defines nanofiber as having at least one dimension of 100 nanometer (nm) or less. The name derives from the nanometer, a scientific measurement unit representing a billionth of a meter [1].Nanofibers are used for several value added applications such as medical, filtration, barrier, personal care, composite, insulation and energy storage. Special properties of nanofibers make them suitable for a wide range of applications from medical to consumer products and industrial to high-tech applications such as aerospace, battery separators, energy storage, fuel cells, information technology, drug delivery system and most importantly in the field of producing tissue engineering scaffolds.Electrospinning is an established method for producing nanofibers from a wide variety of natural and synthetic polymers. The technique combines the design principles of living organisms and modern engineering with the development of viable substitutes of human tissues such as skin, bone, muscle, cartilage and even cardiovascular and neuronal structures. Progress in this field is done via scaffolds implementing with a variety of bioactive molecules to balance cell proliferation and differentiation [2]. The scaffold should have required properties with uniform diameter to help cell in growth. The use of degradable scaffolds has the opportunity for full restoration of the vital tissue.The electrospinning phenomenon itself involves basic and significant issues in polymer science for solution dynamics, in which viscoelastic parameters, surface tension and electro conductivity etc are important factors for the successful spinning of nanofibers. The solvent performs also crucial roles in electrospinning.Polycaprolactone (PCL) is used in this research for producing tissue engineering scaffold. PCL is a biodegradable polymer with potential applications for bone and cartilage repair. The polymer has some advantages over other polymer and is more stable in ambient conditions. It is also less expensive and readily available in large quantities. The scaffolds produced by using PCL matched the design very well, had compressive strength and modulus value within the range of trabecular bone and supported the in-growth of bone in an in vivo model [3].
2. Experimental Details
2.1. Raw Materials
- Polycaprolactone (PCL) is a biodegradable polyester with a low melting point of around 60℃. PCL is prepared by ring opening polymerization of ε-caprolactone using a catalyst.
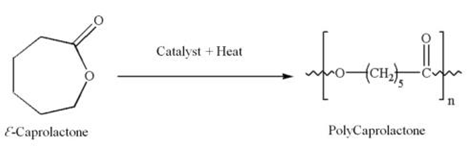 | Figure 1. Ring opening polymerization of ε-caprolactone to polycaprolactone |
2.2. Trifluoroethanol (TFE)
- 2,2,2-Trifluoroethanol is an organic compound with the formula CF2CH2OH, which is used as solvent for producing a scaffold by electrospinning of PCL. It is also known as TFE or Trifluoroethyl alcohol. This colorless, water-miscible liquid has a smell reminiscent of ethanol. Due to the electronegativity of the trifluoromethyl group, this alcohol exhibits a stronger acidic character compared to ethanol. Thus, TFE forms stable complexes also with heterocycles (e.g. THF or pyridine) through hydrogen bond. Industrially trifluoroethanol is used as a solvent for nylon as well as in applications of the pharmaceutical field [5].
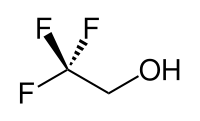 | Figure 2. 2,2,2-Trifluoroethanol |
2.3. Electrospinning Technique for the Production of Tissue Engineering Scaffold
- The electrospinning apparatus for the production of the electropsun PCL reported in this experiment, is set up vertically as shown in figure 3. Three basic components are required to set up the electrospinning process: a syringe with a metal spinneret of small diameter, a high voltage supplier and a rotating collector [6, 7, 8].
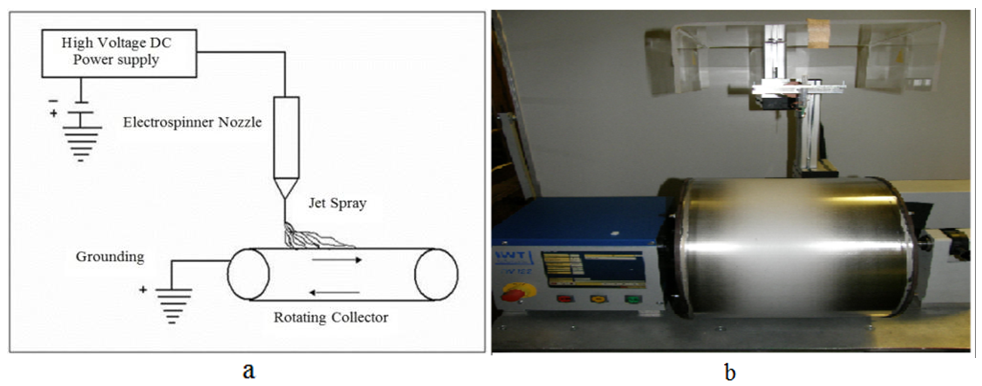 | Figure 3. a) Schematic representation of electrospinning and b) a set-up of electrospinning |
2.4. Parameters of Electrospinning
- High voltage power supply is the most important parameter for electrospinning, i.e. electron moves through the organic solution like polarity and fibers produced like the rays of light. It has been shown that higher voltage produced jets with larger diameters [9].
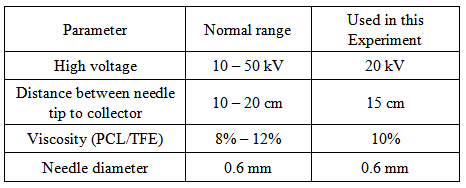
- Viscosity of the solution is also very important to produce nanofibers. The fiber diameter and morphology during spinning of polymeric fibers are affected by the viscosity of the polymer solution. It has been observed that there is no continuous fiber formation with very low viscosity and with very high viscosity it is difficult in the ejection of jets from polymer solution. Thus, there is a requirement of optimal viscosity of polymer solution for electrospinning. Sukigara et al. 2003 have shown the effect of viscosity is significant when electrospinning is done from the melt. To get the required viscosity 3g PCL is mixed with 27g TFE in this experiment. Few experiments have been done by varying the distance between the tip and the collector to control the fiber diameters and morphology. It has been observed that a minimum distance is required for the fibers to get sufficient time for drying properly before reaching the collector [12]. Beading has been observed, if the distance is either too close or too far [13, 14]. The parameters mentioned in the table are selected for this experiment.
2.5. Measurement of Flow Rate
- It is important for an electrospinning process to maintain the required parameter. For this purpose the flow rate is observed for an hour, which is shown in figure 4. The flow rate should be constant during electrospinning.
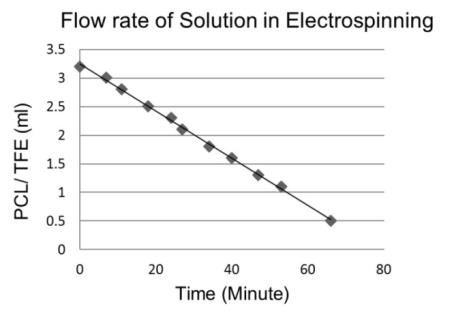 | Figure 4. Measurement of flow rate of solution |
2.6. Sterilization Method
- There are three methods for sterilization such as Gamma radiation, Heating and Chemical process. For conservation and sterilization of our produced scaffold samples, ethanol solution (99%) has been used. Ethanol is used to purify the produced scaffold. But it does not change the morphology of the nanofibers. Therefore some tests have been done to prove that ethanol has no effect on the morphology of scaffolds. That’s why the produced PCL scaffold has been cut into 12 pieces. One was the reference without ethanol treatment (indicated in figure 5 by R) and others are immersed into ethanol with the time difference of five minutes. Sterilization has done in one hour.
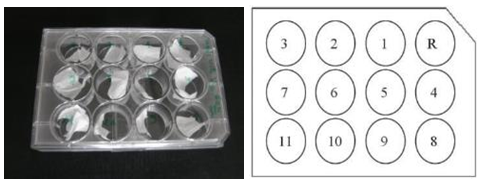 | Figure 5. Electrospun PCL scaffold immersed in ethanol |
3. Result
3.1. Analysis of Produced Scaffold by Microscopy
- The characterization of the morphology of PCL nanofibers is carried out by scanning electron microscopy. The diameters of the fibers are analyzed by the Axio Vision Rel 4.8 program. To evaluate the diameters of fibers, every figure is divided into 3 regions horizontally and 4 regions vertically as shown in figure 6. In every region, the diameters of five fibers are measured in nm scale. The diameter of 60 fibers is measured in every SEM image. This procedure is maintained for all the SEM images.
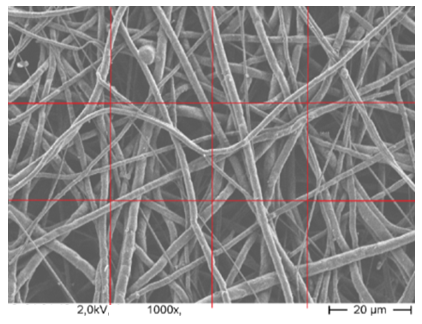 | Figure 6. Measurement procedure of nanofiber diameter |
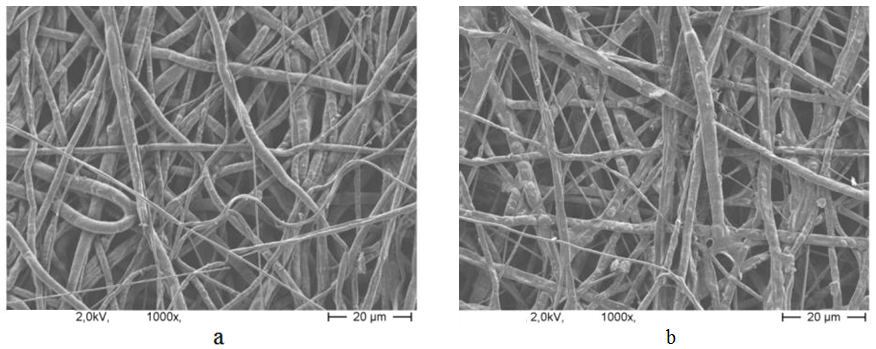 | Figure 7. SEM image of PCL nanofibers sample (1000X magnified); a) without ethanol treatment, b) with ethanol treatment |
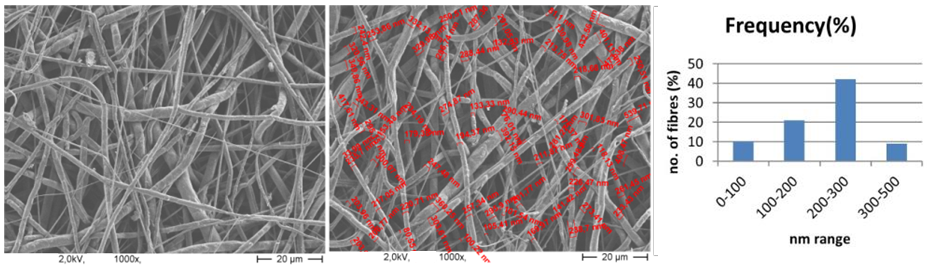 | Figure 8. SEM image of PCL nanofibers (1000X magnified) and range of fiber diameter |
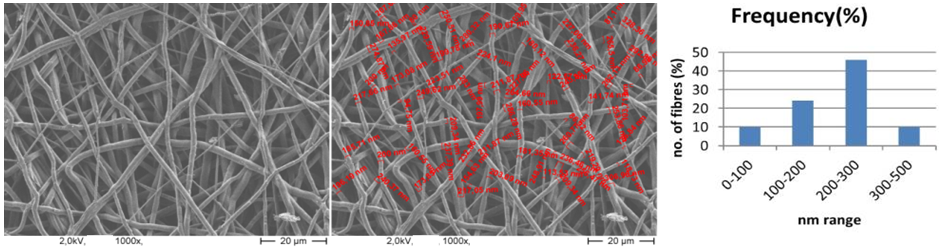 | Figure 9. SEM image of PCL nanofibers (1000X magnified) and range of fiber diameter |
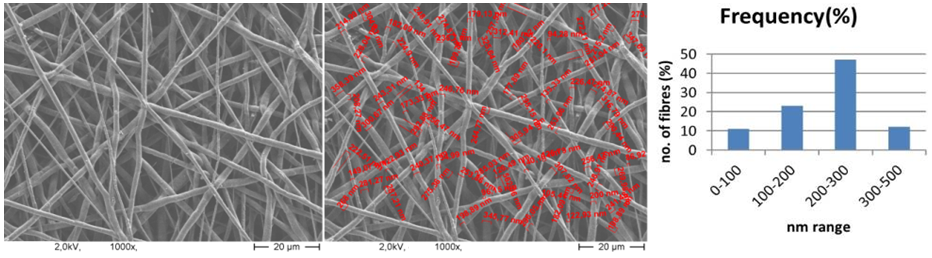 | Figure 10. SEM image of PCL nanofibers (1000X magnified) and range of fiber diameter |
4. Discussion
- The flow rate of the polymer solution from the syringe influences the jet velocity and the material transfer rate. Yuan et al., 2004 have showed that a lower feed rate is more desirables as the solvent will get enough time for evaporation. Thus, there should always be a minimum flow rate of the spinning solution.The polycaprolactone/trifluoroethanol flow rate through the needle of the syringe during the production of nanofibers is low i.e. 0.038ml/min, which is very important for the uniformly distribution of fibers.Few studies have also investigated to find out the relationship between solution flow rate on fiber morphology (Megelski et al., 2002; Zong et al., 2002). High flow rates result in beaded fibers because of unavailability of proper drying time prior to reaching the collector (Yuan et al., 2004; Kim et al., 2005). So, the lower flow rate i.e. 0.038 ml/min is favourable for getting uniform diameter of nanofibers.Figure 7 (a) showed the SEM image of PCL scaffold, which is not treated with ethanol i.e. the reference specimen. The SEM image of PCL scaffold, which is treated with ethanol for 30 minutes shown in figure 7 (b). It is observed that the morphology of nanofibers is almost same in both the SEM images. So it can be said that ethanol has not significant effect on the structure of nanofibers.Figure 8 showed that More than 40% of the fibers in scaffold have been produced with the diameter of 200 to 300nm. Near about 20% of the fibers are produced with the diameter of 100 to 200nm. Less than 10% of the fibers are produced with the diameter of 0 to 100 and 300 to 400nm. Figure 9 and 10 showed that the diameters of the produced fibers are increased a lit bit after treating with ethanol. The diameters of maximum nanofibers are found in the range of 200 – 300 nm. The diameter of nanofibers between 200 and 400nm are found in different research work for different types of tissue engineering applications [10, 11]. The SEM images also showed that the PCL nanofibers have been uniformly distributed in the scaffold, which is very important for cell adhesion in human body.
5. Conclusions
- In recent years electrospinning has gained widespread interest as a potential polymer processing technique for application in tissue engineering. It is a very simple and versatile method for creating polymer based high functional and high performance nanofibers. This technique allows for the production of polymer fibers with diameter on the nanometer scale. The objective of this research is to produce a tissue engineering scaffold from PCL and observe the effect of parameters on the fiber morphology and discuss the application and impact of electrospinning on the field of tissue engineering. It is observed that a uniform scaffold can be produced by using electrospinning technique with the help of PCL. It is also found that ethanol does not have significant effect on the morphology of scaffold, when the nanofiber is sterilized with ethanol. In the future it is very important to apply this technique for the production of nanofibers from different types of polymers.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML