Khalid Pasha 1, John A. Taylor 2
1Textile Engineering Department, NED University of Engineering and Technology, University Road, Karachi, 75270, Pakistan
2Technical Director, Colour Synthesis Solutions Ltd, Delaunays Rd, Manchester M9 8ZS, U.K
Correspondence to: Khalid Pasha , Textile Engineering Department, NED University of Engineering and Technology, University Road, Karachi, 75270, Pakistan.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Abstract
Two monofunctional reactive dyes were synthesized containing a monochlotriazinyl reactive group along with an alkylthio group as second leg in place of usual amino group. Another similar monochlorotrisinyl dye but with usual amino group as second leg was also syntheized as control and both the dyes were characterized. The hydrolysis reactions of both the dyes were studied in alkaline conditions at 60℃ temperature. Rate constant of hydrolysis reactions of both the dyes were determined by high performance liquid chromatography technique. It was found that rate of hydrolysis reaction of monochlorotriazinyl dye having an alkylthio group as second leg substituent was faster than the monochlorotrizinyl dye having an amino group substituent.
Keywords:
Reactive Dyes, Kinetic Study, Monochlorotriazinyl Dye, Alkylthio Group, Hydrolysis
Cite this paper: Khalid Pasha , John A. Taylor , Kinetic Study of Hydrolysis Reaction of Monochlorotriazinyl Reactive Dyes Containing an Alkylthio and Alkylamino Substituent, International Journal of Textile Science, Vol. 3 No. 2, 2014, pp. 29-32. doi: 10.5923/j.textile.20140302.02.
1. Introduction
Dichloromethylmercapto- [1, 3, 5] triazine (Figure 1) has been known and used in chemical industries especially in agrochemicals for many years. 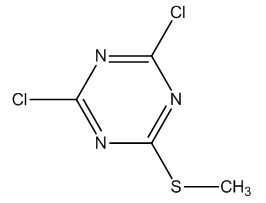 | Figure 1. Dichloromethylmercapto- [1, 3, 5] triazine |
Chlorotriazine compounds of the type shown in figure 1 have been used in making special selective herbicides [1] like Ametryn [2] and Desmetryne [3, 4] shown in Figure 2. 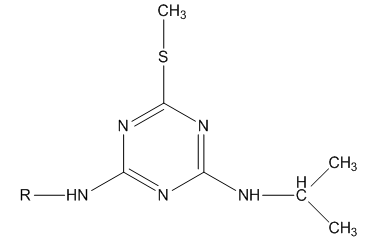 | Figure 2. When R= C2H5, Ametryn; R=CH3, Desmetryne |
Calculations of the charge distribution over the various unsubstituted nitrogen heterocycles (pyridine, pyrimidine) showed that the largest positive charge recorded in the series is on the carbon atom of the s-triazine ring [5]. Carboxymethylthiol-triazine and carboxyethylthiol-triazines studied by Lehr [6] and another study by Smith et al [7] on cysteamine crosslinked triazine systems to give tri- and tetra funcional chlortriazinyl dyes were also reported.In most commercial dyes the second chlorine atom of the trichlorotriazine is usually replaced by an amino substituent either to incorporate another reactive group or to attach another chromophore. Nitrogen is more electron withdrawing than sulphur by an inductive effect. Although both Nitrogen and Sulphur atoms have a lone pair of electrons but sulphur being a larger atom as compared to Nitrogen cannot donate its lone pair of electrons as effectively as Nitrogen. Therefore, the overall activity of remaining chlorine atom of triazine ring could be increased if the nitrogen atom attached as second leg atom be replaced with sulphur. To evaluate this concept a model dye (Dye 1) was synthesized together with Dye 2 (Figure 3) as control and their rates of hydrolysis in dilute (0.05 M) aqueous sodium carbonate were determined. 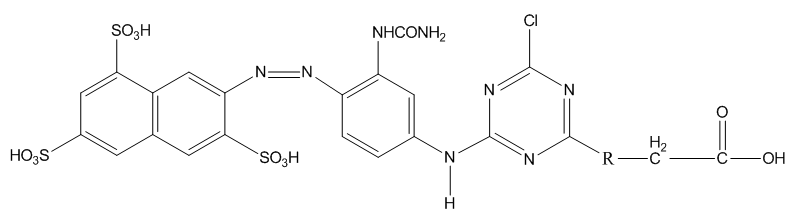 | Figure 3. When R=S, Dye 1; R=NH, Dye 2 |
2. Experimental
Two techniques i.e. FAB (fast atom bombardment) and MALDI (matrix assisted laser desorption ionisation) were used to obtained Mass spectra. Aluminium plates coated with silica gel, 60 F254 (Merck) were used for Thin layer chromatography. A Brűker DPX 300 instrument equipped with a proton/carbon, z-gradient, dual probe with an automatic sample changer Proton was used for NMR spectra of samples. Solvent used was deuterium oxide > 99.9 atom% D, with tetramethyl silane as an internal standard. C.I. Reactive Orange 86, thioglycollic acid, glycine, sodium hydroxide and sodium carbonate used were available commercially. Titanous chloride titration method [8] was used for estimation of strength (concentration) of any given dyestuff.
2.1. Synthesis of Dyes
Two dyes (Dye 1 and Dye 2) were synthesized as detailed below. The pH of the reaction mixture and temperature in both the synthetic routes were different. Although the electrophile, dichlorotriaizine dye, was the common starting material, in one case the attacking nucleophile was thiol (thioglycollic acid) and in other it was an amino group (glycine). The higher pH utilized in reaction with glycine was necessary in order to ensure that sufficient free, unprotonated, amine was present to allow reaction in a reasonable timescale.
2.1.1. Synthesis of 3-ureido-4-(3,6,8-trisulphonaphth-2-ylazao)-N-(4-chloro-6-carboxymethylthio-s-triazin-2-yl)aniline (Dye 1)
Thioglycollic acid (0.92 g, 0.01 mole) was added portion wise to a stirred solution of C.I. Reactive Orange 86 (0.01 mole, wt. 9.03, M.I. 903) in water (100 ml) at 10-12℃, pH of the reaction mixture was maintained at 7 to 7.5 with sodium carbonate solution. After the completion of the addition of thioglycollic acid (10 minutes), the temperature of the reaction mixture was slowly raised to 20℃ and the mixture was stirred with control by t.l.c. and h.p.l.c. After the completion of reaction (60 minutes) as judged by h.p.l.c. the product was precipitated by the dropwise addition of ethanol (150 ml) the solid was collected and dried under vacuum, Rf value 0.34, h.p.l.c. retention time 2.66 minutes (M+H+) sodium salt peak 772, molecular weight 748.5, yield 5.25 g, 43 %, M.I. 1211, strength 62%.
2.1.2. Synthesis of 3-ureido-4-(3,6,8-trisulphonaphth-2-ylazao)-N-(4-chloro-6-arboxymethylamino-s-triazin-2-yl) aniline (Dye 2)
Glycine (1.50 g, 0.02 mole) was added directly to a stirred aqueous solution (100 ml) of C.I. Reactive Orange 86 (18.06 g, 0.02 mole) at room temperature. The temperature was raised to 35℃ and pH was raised to 9.5 with sodium carbonate solution (10% w/v) and maintained at 8 to 9.5 during the course of the reaction. The reaction mixture was stirred with control by t.l.c. and h.p.l.c. for 4 hours after which time the reaction was complete as judged by h.p.l.c. The product was precipitate by drop wise addition of ethanol (450 ml), collected and dried under vacuum. Rf value 0.32, h.p.l.c. retention time 2.78 minutes, yield 16.0 g, 63 %, M.I. 1275, strength 56.40%..
2.2. Kintetic Experiments
The kinetic experiments were performed under pseudo-first order conditions i.e. in a large excess of hydroxide ion [9]. Hydrolysis of model dyes (Dye 1 and Dye 2) were performed in a solution of 5 x 10-3 moles of Analar sodium carbonate (99.5%) in 100 ml distilled water at 60℃. 1-naphthalenesulphonic acid, sodium salt (0.1 g, 4.34 x 10-4 moles) was added to act as an internal standard reference for h.p.l.c. Sodium carbonate and 1-naphthalene sulphonic acid. Sodium salt were added to distilled water (100 ml) in a two necked round bottomed flask, fitted with suba-seal stoppers to avoid loss due to evaporation. The temperature was maintained at 60℃±I with a thermocouple controlled stirrer hotplate. At this temperature, 5 x 10-5 mole of the model dye was added and after timed intervals 1 ml aliquots were removed and quenched by adding to excess buffer solution of pH 7, room temperature. Samples collected were analysed by h.p.l.c. and the formation of hydroxyl product and disappearance of starting monochloro triazine were monitored quantitatively by comparison of h.p.l.c. peak area against the internal standard to minimized handling and instrumental errors.
3. Results and Discussions
3.1. Kinetic Studies
Dyes were dissolved in an aqueous solution of sodium carbonate at a constant temperature and the rate of disappearance of monochlorotriazine parent dye and the formation of hydrolysed product were measured quantitatively with HPLC against an internal standard. | Table 1. Kinetic study data of Dye 1 |
| | Time (min.) | Dye 1 (% area) | Log c/c-x | | 0 | 100 | - | | 10 | 98.55 | 0.0043 | | 20 | 97.57 | 0.0086 | | 40 | 93.39 | 0.0293 | | 50 | 92.05 | 0.0334 | | 80 | 89.81 | 0.0453 | | 110 | 84.11 | 0.0718 | | 140 | 78.37 | 0.1038 | | 170 | 75.75 | 0.1205 |
|
|
Data recorded by HPLC as showed in Table 1 for Dye 1 and Table 2 for Dye 2 were used to calculate the rate of hydrolysis of the sample dyes by assuming that the area under the peak relative to the internal standard is directly proportional to the amount of the product i.e. concentration. Decrease in concentration (peak area) of the starting material against internal standard, and the increase in concentration of hydrolysed dye recorded. | Table 2. Kinetic study data of Dye 2 |
| | Time (min.) | Dye 2 (% area) | Log c/c-x | | 0 | 100 | - | | 10 | 98.90 | 0.0043 | | 20 | 98.51 | 0.0043 | | 40 | 95.53 | 0.0170 | | 50 | 94.22 | 0.0253 | | 80 | 91.83 | 0.0334 | | 110 | 87.10 | 0.0569 | | 140 | 84.62 | 0.0718 | | 170 | 81.99 | 0.0827 |
|
|
3.2. General Derivation of Rate Equation
They hydrolysis of a chlorotriazine dye in aqueous hydroxide can be represented by equation 1. 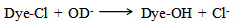 | (1) |
If the concentration of hydroxide ions present in the reaction medium is effectively constant (due to being present in a large excess), the reaction rate at constant temperature (60℃) can be represented by equation 2.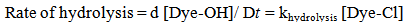 | (2) |
where [Dye-OH] and [Dye-Cl] are the concentrations of the hydrolysed and monochlorotriazine dyes respectively. If the initial concentration of the chlorotriazine dye is “c” at time “t”, “x” moles of chlorotriazine would have decomposed and consequently ”x” moles of hydrolysed dye would have formed. The concentration of the remaining chlorotriazine at that time is equal to “e-x”, and the rate equation becomes: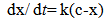 | (3) |
and after integration;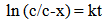 | (4) |
which can be converted into the base 10 logarithm;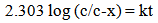 | (5) |
This equation describes a straight line of type;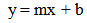 | (6) |
with slope “m” and with intercept “b” equal to zero. For a first-order reaction, a plot of “log c/c-x” against time “t” will be a straight line whose slope is “k/2.303” and from which the rate constant “k” of the hydrolysis reaction can be calculated. On plotting the “log c/c-x” values for Dyes 1 and 2 against time “t” (in minutes), straight lines were obtained (Figure 4 and Figure 5), from which rate constants were calculated. Rate constant for hydrolysis of Dye 1 and Dye 2 were 3.03 x 10-4 and 2.17 x 10-4 L mol-1 min-1 respectively. The results obtained indicate that the rate of hydrolysis of Dye 1 (with sulphur substituent) is faster (1.4 times) than that of Dye 2 (with amino substituent). Although the reactivity difference was not huge, however it was in accordance with expectations. 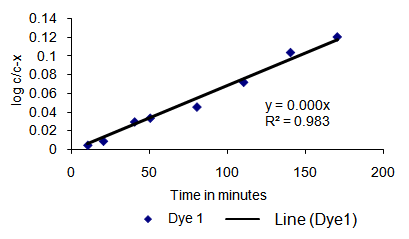 | Figure 4. Log c/c-x versus time in minutes for Dye 1 (with sulphur group) |
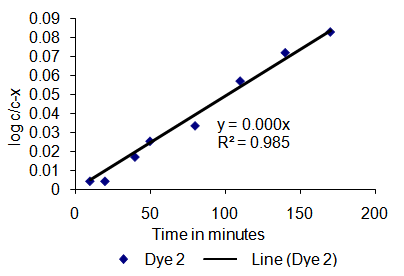 | Figure 5. Log c/c-x versus time in minutes for Dye 2 (with amino group) |
4. Conclusions
The reactions of two monofunctional reactive dyes with water at alkaline pH have been studied kinetically by utilizing High Performance Liquid Chromatography technique method and their rate constants were measured. Rate of hydrolysis of monochlorotriazinyl dye having alkylthio substituent as second leg was slightly faster than monochlorotriazinyl dye having amino group substituent as second leg at same reaction conditions. From the results of this rate study it appeared that the presence of an alkylthio substituent in place of an amino substituent on a triazine ring resulted in enhanced reactivity of the remaining chlorine atom of the triazine ring.
References
| [1] | Ciba, United States P 2,909,420 (1959). |
| [2] | Martin H and Worthing C R, Pesticide Manual, 4th Edition., British Crop Protection Council, 1974, 12. |
| [3] | Ciba, Belgian P 714,992 (1972). |
| [4] | Ciba, British P 1,191,585 (1972). |
| [5] | Peacock T E, Electronic properties of aromatic and heterocyclic molecules, Academic Press, London, 1965,103. |
| [6] | Lehr F., Synthesis and application of reactive dyes with heterocyclic reactive systems, Dyes and Pigments, 1990; 14:239-262. |
| [7] | Smith B, Berger R, and Freeman H S, High affinity, high efficiency fibre-reactive dyes, Color. Technol., 2006; 122:187-193. |
| [8] | Murtagh V, Taylor J. A, A simple titrimetric method for the estimation of reactive dye fixation on cellulosic fabrics, Dyes and Pigments, 2004; 63: 17-22. |
| [9] | Renfrew A H M and Taylor J A, Reactive dyes for cellulose. Concurrent methoxide-hydroxide reactions of triazinyl reactive systems: a model system for assessment of potential fixation efficiency, JSDC, 1989;105: 441-445. |



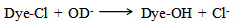
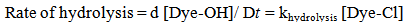
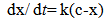
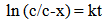
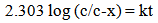
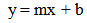


 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML