-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Textile Science
p-ISSN: 2325-0119 e-ISSN: 2325-0100
2013; 2(4): 121-125
doi:10.5923/j.textile.20130204.06
Effect of Nano Zinc Oxide onto Polypropylene Properties Yarns I-UV Protective Polypropylene /Nano Zinc Oxide Yarns Using Compounding Melt Extrusion and Spinning Technique
S. M. Gawish, A. M. Ramadan
National Research Centre, Textile Division, Tahrir street, Dokki, Cairo, Egypt
Correspondence to: S. M. Gawish, National Research Centre, Textile Division, Tahrir street, Dokki, Cairo, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
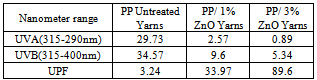
In this study, we have investigated the preparation of UV-Protective PP/nano ZnO composite yarns by the meltextrusion -spinning method. The produced composite yarns contain nano ZnO particles at (1,2,3% ) loadings. The results have shown that the used PP at all nano particles combinations has good spinnability at 230℃ using compounding and melt –spinning processes. The yarns containing 3% nano ZnO is UV protective since UVB, UVF are 5.34 and 89% compared to the untreated yarns which have attained 34.57 and 324% respectively.
Keywords: UV Protection, UV Absorber, Composite Yarns, Polypropylene, Nano Znc Oxide
Cite this paper: S. M. Gawish, A. M. Ramadan, Effect of Nano Zinc Oxide onto Polypropylene Properties Yarns I-UV Protective Polypropylene /Nano Zinc Oxide Yarns Using Compounding Melt Extrusion and Spinning Technique, International Journal of Textile Science, Vol. 2 No. 4, 2013, pp. 121-125. doi: 10.5923/j.textile.20130204.06.
Article Outline
1. Introduction
- The use of nanotechnology in the textile industry has increased rapidly, mainly due to the fact that the used conventional methods to impart different properties to fabrics often do not lead to permanent effects and the fabrics will lose their functions after laundering or wearing. Nano particles can provide higher durability for the treated fabrics, compared to the conventional ones, because nano particles possess large surface area and high surface energy that ensure better affinity for fabrics and lead to increase the durability of the textile functions. Polypropylene (PP) fibers are the most widely used synthetic fibers in textile industry, especially for their various application fields. In fact, some advantages of these fibers include cheapness, lightness, high chemical strength and high absorbency, have made them suitable for many demands, such as carpets, automotive interior design, films, packaging, cover stock, cables, napkins, wipes. In particular, they are used for sanitary applications, such as surgical masks, diapers, filters, hygienic bands, which need to display antibacterial effects.On the other hand, nano Zinc Oxide particles are widely used in different areas for their unique photo-catalytic, electrical, electronic, optical, dermatological, and antibacterial properties[1-7]. In these applications, the nano particles need to be well dispersed homogeneously in the different matrices and a number of new synthetic fabrics have been developed in order to prevent particles agglomeration and increase the stability of nano ZnO particles dispersions[8-9]. Nano `ZnO has three key advantages[10]. It is a semiconductor, with a direct wide band gap of 3.37 eV and a large excitation binding energy (60 meV). It is an important functional oxide, exhibiting excellent photo-catalytic activity. For its noncentral symmetry, ZnO is piezoelectric, which is a key property in building electromechanical coupled sensors and transducers. Also, ZnO is bio-safe and biocompatible and can be used for biomedical applications without coating. With these three unique characteristics, ZnO could be one of the most important nano materials in future research and applications.Literature review revealed that the application of nano particles in textile materials is the objective of several studies aimed of producing finished fabrics with different performances. For example, nano Ag has been used for imparting antibacterial properties[11,12], nano titanium dioxide TiO2 for UV-blocking and self-cleaning properties [13-15], ZnO nano particles for antibacterial and UV-blocking properties[16]. Metal oxide nano particles are more preferable than nano silver due to cost effectiveness. In fact, ZnO and TiO2 are non-toxic and chemically stable under exposure to both high temperature and capable of photo-catalytic oxidation. Furthermore, nano particles have a large surface area to-volume ratio that results in significant increase of the effectiveness in photo- catalytic oxidation activity when compared to bulk properties[17].TiO2 and ZnO nano metals have unique properties such as higher stability, long lasting, safe, broad-spectrum anti-biosis[18] and are applicable in many fields such as self-cleaning, anti-bacterial agent, UV-protecting agent, environmental purification, water and air purifier, gas sensors, and high efficient solar cells[19].To enlarge the light absorption range of TiO2 from UV to visible spectrum and notable increasing the efficiency of photo catalytic activities, in many applications, it has been investigated the addition of noble metals, such as Au or Ag, forming metallic nano TiO2 composites[20, 21], or the reaction with hydroxyl propyl cellulose (HPC), forming non-metallic nano TiO2 composites[22].To get an optimal self-cleaning ability ,it is necessary to have a super- or ultra-hydrophobic treatment with a very high water contact angle (above160°) and a very low roll-off angle, increasing, in this way, surface roughness with a larger geometric area and easily removing particles of all kind (dirt, stain, oil, mud etc.) adhering to fabric surface. Self-cleaning textiles could find wide use in medical and sports applications. To obtain a self-cleaning property, it is possible to coat the textile materials with metallic ions or metallic compounds, like TiO2 or ZnO (with different band gaps, respectively, 3.2 and 3.37 eV), that react with organic matters (dirt, stain, bacteria, pathogenic fungi), obtaining a sterilizing effect and the discoloration of stains[23]. Metal oxides, in fact, such as TiO2 ZnO, MgO and CaO are very interesting because they result to unstable and aggressive against microorganisms under specific environmental conditions but, at the same time, generally safe to human beings and animals[24]. In particular, titanium dioxide NPs, mostly produced by sol-gel processing method[25], are very interesting especially for their photo catalytic activities. In fact, their molecules, activated by an energy of UV-light higher (with λ ≤ 387.5 nm in anatase form)[26], its band gap, emit an electron (e-) that, jumping from the valence band to the conduction band and combining itself with O2, in the air or water, forms active O2- (photo-oxidation), while the electron hole (h+), reacting with water, generates hydroxyl radicals (OH-). O2- and OH- catalytically dissolve organic compounds (dour molecules, bacteria and viruses), respectively, into CO2 and H2O (photo-catalytic effect by inverse redox reaction) [27, 28].In the following investigation PP/ nano ZnO (1-3 % ) composite yarns were synthesized using compounding apparatus and the extrusion - melt spinning process. The ultra violet protective properties of the compressed yarns are evaluated by their UV protection factor (UPF) which indicates the degree of protection against UV radiation.
2. Experimental
2.1. Materials
- Isotactic polypropylene chips (PPH90E9) were supplied from Total Petro Chemicals Co. in Europe, specific gravity 0.902 and melting point 171℃, nano Zinc Oxide powder was purchased from Zano Co. Belegium, (d ~ 20 nm, MW 81.37g/mol, mp 197.9℃, density 5.47 g/cm3). The lubricating oil for the yarns is crosanol 1-PP11(20 oil /80 water).
2.2 .Methods
2.2.1. Synthesis of Nano ZnO / PP Yarns
- Synthesis master batch of nano ZnO/PP chipsThe compounding of nano ZnO to PP was done in a thermo prism PTW-16 (Haake Co. England, Fig1 ) ,which is a twin screw extruder and the calculated weights for (1,2,3% loadings nano ZnO ) is added in a hopper (ie 90 gm. nano ZnO are mixed to one kilo PP, added in the other hopper ) and extruded in several temperature (180 to 220℃) in the form of thin strips which are then cut into master chips.
 | Figure 1. Compounding ( Haake Co. , England) machine for master batch for nano ZnO/ PP |
 | Figure 2. Extrusion and melt spinning machine (Spin Boy1) for nano ZnO/PP |
2.2.2. Spinning Process
- The previous prepared master batch for 3% nano ZnO loading is mixed to two kilo PP in the spinning equipment (Fig.2).The machine used for the research was a Spin boy1 It contains seven extruded zones (195,200 to 220℃).The melt was extruded through the spinnerets after which the fibers were transported over the Godet rolls. The fibers are drawn with a constant draw ratio of 3 and a speed of 200m/mn to 600m/mn and each fiber contains 80 filaments of 148 denier.
2.3. Characterization Properties of Nano ZnO / PP Composite Yarns
2.3.1. Determination of Zinc Oxide Content
- Fibers were cut into small pieces with a stainless steel scissor, weighed with accuracy to 0.00001 g, and were placed into a Pyrex beaker and then combusted in a muffle furnace with slowly raising the temperature by 10℃ every 30 min to a final combustion of 550℃ for 1 h. The residue was dissolved in 10 ml HNO3 and filled with distilled water to 100 ml in a volumetric flask, rinsed several times with deionized water and then brought to volume. The solution was diluted 10 times and analyzed[29] on a Varian 820 ICP - Mass Spectrometer to measure zn in the solution, which is then converted to ZnO content.
2.3.2. UV Protective Properties of Compressed Yarns
- PP/nano ZnO composite yarns were pulverized to a fine powder and compressed to a thin transparent round disc under the jaws of a mangle and used as samples for the measurement of UPF value .The ability of the coated fiber to block UV light is given by the ultraviolet protection factor (UPF) value. The measurement of UPF values was performed in UV/Visible Spectrophotometer 3101 PC with a software version, using an integrating sphere loaded with the fabric sample from 290 nm at an interval of 10 nm.The measurements of the UV penetration characteristics of the compressed yarns sample were carried out in the range of 290-400 nm by using the UV penetration and protection measurements system. Before measurements, relative humidity for 24h is done for the sample. During the measurements, four scans were obtained by rotating the sample 90o each time and the spectral data were recorded as the average of these four scans. The equation used by the software to calculate the UPF value for a flat, tensionless dry fabric is as follows[30].
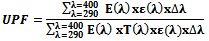 Where E(λ) is the solar irradiance (W/m-2nm-1) measured ; ε(λ) is the arythemal action spectrum; Δ λ is the wave length interval of the measurements; and T(λ) is the spectral transmittance at wavelength λ290. The percentage blocking of UVA (315-400nm) and UVB(315-290nm) was calculated from the transmittance data .
Where E(λ) is the solar irradiance (W/m-2nm-1) measured ; ε(λ) is the arythemal action spectrum; Δ λ is the wave length interval of the measurements; and T(λ) is the spectral transmittance at wavelength λ290. The percentage blocking of UVA (315-400nm) and UVB(315-290nm) was calculated from the transmittance data .3. Results and Discussion
3.1. Real ZnO Filler Content of PP Yarns
- The analyses of the real Zn in PP / nano ZnO yarns is found to be 2.76% Zn (3.44% ZnO) the content of ZnO in this sample is 3% the excess amount of ZnO which is 0.44% is probably due to the moisture regain to the fibers which were not dried before analysis.
3.2. Evaluation of Nano ZnO/PP Compressed Yarns for UV Protection and UV Transmission %
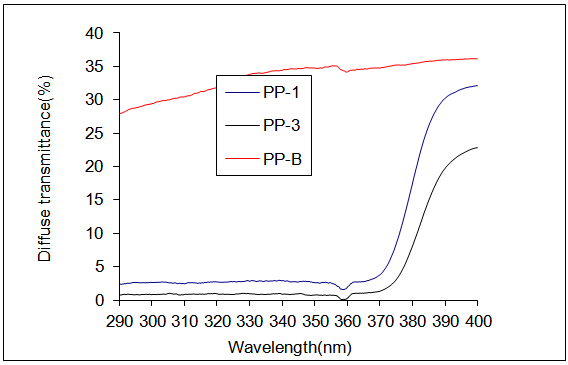 | Figure 3. UV / visible transmission spectra of polypropylene yarns for 1-the blankyarn , 2- PP/1%ZnO, 3-PP/3%ZnO |
|
4. Conclusions
- The production of UV protection PP/nano ZnO composite yarns was done by mixing a concentrated master batch with PP at 1-3% loading using compounding and melt extrusion, spinning methods. The ZnO content of the yarns were analyzed showing a real content of the same value as the experimental amount. This indicates that no loss of nano ZnO during the process was done by agglomeration of particles on the filters o f the apparatus. The nano ZnO coated PP is found to have the UV blocking property, about 89.6 % of the incident UV light of sample containing 3% nano ZnO was absorbed due to the coating compared to 3.24% for the untreated blank.
ACKNOWLEDGEMENTS
- The authors would like to thank Prof.Eric Devaux, Director of Gemtex lab and Engineer Guillaume Lemort for the fabrication of Poly propylene com- posite yarns. Also the financial scholar grant for one month given by Campus France in Paris for Prof. Gawish and which was initially supported by Mr Guillaume Adoque, Director of grants at the French Cultural Centre in Cairo, is greatly appreciated.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML