Ing. Iba Gaye Ph.D (SENEGAL)
Technical University of Liberec, Department of materials science
Correspondence to: Ing. Iba Gaye Ph.D (SENEGAL), Technical University of Liberec, Department of materials science.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Abstract
This work presents a comparison of the properties of conventional and organic cotton, using apparatus which are available at the Faculty of Textile and TUL, an analysis of cotton was made with the raster electron microscope SEM VEGA TS 5130, the physicochemical properties with GC / MS, analytical method of gas chromatography, the mechanical properties of both types of cotton were studied in Labor dynamometer test, strength was measured using Pressley Tester instrument response and fibers on the dynamic mechanical analyzer DMA., cotton maturity was determined using a polarizing microscope, using the apparatus Micronaire for fineness, staple length with staple diagram, thermal properties with the differential scanning calorimeter (DSC) and thermo gravimetric analyzer TGA. To detect the presence of pesticides in samples of cotton, gas chromatography with mass was employed and the results were compared to those obtained from the gas chromatography mass spectrum GC / MS. On the basis of these results it was concluded that the gas chromatographic method can be used to detect pesticides in cotton samples and can be applied to all natural fibers of plant origin. In this way, they can be distinguished as organic and conventional cotton, thus contributing to the certification of organic cotton and substances of vegetable origin. Analysis, which is introduced in this thesis, demonstrates difference in conventional and organic cotton from Senegal, Egypt, Russia and India.
Keywords:
Organic, Coventional, Chromatography, Abundance
Cite this paper: Ing. Iba Gaye Ph.D (SENEGAL), Certification of Organic Cotton by Detecting Pesticides on Cotton Fiber, International Journal of Textile Science, Vol. 2 No. 4, 2013, pp. 81-91. doi: 10.5923/j.textile.20130204.02.
1. Introduction
Cotton is the most important natural textile fiber, as well as cellulosic textile fiber in the world, used to produce apparel, home furnishings, and industrial products. World wide about 40% of the fiber consumed is cotton. Cotton is grown mostly for apparel application but it is also a food crop (cotton seed) the major end uses for cotton seeds are vegetable oil for human consumption; whole seed, meal, and hulls for animal. Cotton fibers are seed hairs from plants of the order Malvales, family Malvaceae, tribe Gossypieae, and genus Gossypium. Botanically, there are four principal domesticated species of cotton of commercial importance: hirsutum, barbadense, aboreum, and herbaceum. Thirty - three species are currently recognized; however, all but these four are wild shrubs of no commercial value. Each one of the commercially important species contains many different varieties developed through breeding programs to produce cottons with continually improving properties, faster maturing, increased yields, and improved insect and disease resistance and fibers with greater length, strength, and uniformity.[1]Of all organic fibers, organic cotton is one of the most popular. Organic cotton is grown using methods and materials that have a low impact on the environment. Organic production systems replenish and maintain soil fertility, reduce the use of toxic and persistent pesticides and fertilizers, and build biologically diverse agriculture. Third - party certification organizations verify that organic producers use only methods and materials allowed in organic production. Organic cotton is grown without the use of toxic and persistent pesticides and synthetic fertilizers. In addition, federal regulations prohibit the use of genetically engineered seed for organic farming. All cotton sold as organic in the United States must meet strict federal regulations covering how the cotton is grown.The organic cotton is of great interest for the following reasons:-Price of organic cotton is higher than the price of conventionally grown cotton.- Debt for growers is smaller, because farmers no longer need to buy chemicals.Eliminates the negative effects, such as human and animal poisoning by chemicals and soil toxicity, as well as natural cosmetics, also called organic materials beginning to have a firm place in the shopping area for only environmentally minded consumers. But what really means in the case of bio preposition shirt, towel or baby products. And how to navigate in their marking? Textile industry has long been among the biggest load factors of the environment. Nearly a quarter of all insecticides applied annually is used in the cultivation of cotton. Textile industry has long been among the biggest load factors of the environment. Nearly a quarter of all insecticides applied annually is used in the cultivation of cotton. Other significant amounts of toxic chemicals are consumed annually in the conversion of cotton fiber for shirts, towels, bed linen etc. Neither the textile production of other vegetable and animal fibers, nor the complicated chemical processes of 100% cotton, silk or wool, is far from the idea of the nature of natural materials that we recall at first glance. It is not surprising that many people interested in turning to clothing produced a way for nature and man favorable, even in products labeled as "natural", "bio","eco" or "green" textiles, need to be able to measure and define. pesticides are biologically active compound which control the growth of organism e.g. bacteria, fungi, algae, insects or plants.The market of organic products rapidly increased and how we can attest that product are organic or not that s the big problem.By using method which are underpinned on the latest developed technology i mean nanotechnology, mechatronics and informatics we are able according to this method to identify or detect pesticides on cotton fiber,to give a name, and chemical formula by exciting the molecule in order to dislocate,to transformed in positive and negative charged ions and to identify by its ratio mass to charge (despite the charge of the ion) that s the princip of GC/MS
2. Main Goals
As already stated above, the aim of this work is to compare conventional and organic cotton. Analysis was carried out by experiment, for specific properties of both types of cotton fibers. In addition to these analyses it is urgent to have a scientific way how to certify organic cotton, to find a methodology which would identify pesticides in cotton fibers. Thereby contribute to preventing counterfeits, which is presented as conventional organic cotton. This happens in case of 30% of products from organic cotton. This means identifying the cotton impermissible substances (especially pesticides). Analyses should have explanatory power for both raw cotton and cotton products. In practice, the method to distinguish between organic and conventional cotton does not exist.
3. Start of Art
The cotton plant is a tree or a shrub that grows naturally as a perennial, but for commercial purposes it is grown as an annual crop. Botanically, cotton bolls are fruits. Cotton is a warm weather plant, cultivated in both hemispheres, mostly in North and South America, Asia, Africa, and India (in tropical latitudes). Mostly it is cultivated in the Northern hemisphere. It is primarily grown between 37´8 N and 32´S but can be grown as far north as 43´8 N latitude in Central Asia and 45´ 8 N in main land China. Planting time for cotton varies with locality, from February to June in the Northern hemisphere. The time of planting in the Northern hemisphere is the time of harvest in the Southern hemisphere. Seedlings emerge from the soil within a week or two after planting, 5–6 weeks later flower buds or squares form, and white (Upland cotton), creamy yellow (Pima cotton) to dark yellow blossoms appear in another 3–4 weeks. The time interval from bloom to open boll is about 40–80 days. The open boll lets air in to dry the white, clean, fiber, and fluff it for the harvest. Fiber bundle cross-sections obtained with transmitted light microscopy for natural brown cotton where the presence of material bodies are visible within the lumens of some fibers and for natural green cotton where the fibers are quite immature and are characterized by the presence of suberin in the fiber walls and not material bodies in the lumen.[4]
4. Parameters Seeds and Growing Conditions Pedological Organic and Conventional Cotton
Soils in the region Koungueul city in Senegal, where organic and conventional cotton is grown, is alluvial, sandy, ferric, salty, lithographical leaching.Type of seed: Genealogy (IRMA 1243 PB * 5) - 32 45-859 E 281-789 F - G 440 company ISRA / Senegalese Institute for Agricultural Research / hybrid 1986th type of pyramid. green-red . The capsule medium, seeds and green. harvest 43.9% fiber (10 saws). Capsule Weigt 3.72 g (ISRA). Used fertilizer type NPKSB (14-23-13-5-1) 200kg/ha, pesticide, 3.7 l / ha urea 30kg/ha[2] seeds are the same in these fibers: con1, con2, bio1, bio2.[2]Cotton plant grows in tropical and subtropical conditions, lives a decade and can measure up to ten meter. Cultivation of cotton shows that its size is limited to one to ten meters, to facilitate the collection of cotton and is usually used as an annual plant. During flowering period appear large white or yellow flowers with five petals flowers. For cotton, alternating between wet and dry climate is important for its development, however in the African savanna, where cotton grows, the climate is characterized by wet and dry from April to August. African soil is already rich enough organically, the soil is highly enriched with chemical fertilizers, except that at the end of the growing season, the plants are cut and burnt directly in an area that allows direct recirculation of most nutrients, but also reduces the availability of phosphorus. But at maturity cotton plant deshice rapidly, exposed to sun and air, water contains decreased and give the twist form to cotton fiber which is very important characteristic for spinning.[4]
5. Critical Evaluation of the Literature and Overview of the Current Situation
If cotton can be considered as one of the fibers, which accompanies man throughout life, with the emergence of organic cotton a very serious problem emerges for some experts who have a very suspicious vision or a very simplified analysis of organic cotton. That is to provide kit for the detection of pesticides on cotton , methods based on electrochemical using bio sensors , algae with ultra sound extractor, using FT-IR, which does not give a complete solution for the detection of pesticides. Choosing organic cotton is to contribute to the preservation of our health, especially for children; cotton consumes huge amounts of pesticides that are applied in the range of 5-10 times than other vegetables. But these chemicals are great problem on the environment in the form of contamination of soil, water, food, ground water etc. Another disturbing factor in the cultivation of cotton is also that in third world countries, job and work conditions are disastrous and child labor is used. Certification of organic cotton is done at present under the same cultivation conditions. Some argue that simply pesticides do not exist on cotton as confirmed Institute at Bremen, Germany or BVT Brno(czech republic). In 2009 during a visit to TUL in recommending us to do tests on pesticides immediately after harvest or on cotton, based on their tests on samples of cotton from Senegal, but under the proposed detection methodology. This can detect chemical pesticides. Type of pesticides and pesticide tests were conducted also with the old cotton samples (15 years) of cotton (Syria, Egypt, Indie). It can be confirmed that there is no trace of pesticides on cotton fiber, the methodology developed in this work, today confirms that this method can detect pesticide for cotton, gives a chemical name. and chemical formula of the pesticide.
6. Methodology and Working Practices
The main properties of cotton fiber have been investigated using devices at disposal in the textile faculty and in the technical university TUL.
7. Detection of Pesticide in Organic and Conventional Cotton Using Gas Chromatography GC / MS
As mentioned earlier, there are many chemicals in large quantities, which are not friendly to the environment, but also hazardous for physical chemical method is based on determining the mass of atoms, molecules and their fragments after transfer to the positively and negatively charged ions. It is an ideal method for finding the chemical structure of pesticides present in cotton fibers. Gas mobile phase is used here only for analysis and effects on the stationary phase. GC-MS characterizes especially effective and rapid separation of complex mixtures and works with small amounts of samples, using relatively simple equipment. Gas chromatography with mass spectrum retains priority status for the analysis of pesticides. There is requirement of a high selectivity of the separation process, the separation should occur as little as possible to analyze ions, at present, gas chromatography with a mass spectrum is widely used. The combination of GC-MS represents a very promising combination of effective separation method with highly sensitive detection used in the detection of pesticides in cotton samples from Senegal, from Egypt, Russia and Indie. Auto sampler is placed on top of the GC capillary column of fused silica. GC converts directly to the assembly line, in ion traps, where the samples are introduced or injected either manually or by using the auto sampler people.Gas chromatographic method is used for the separation and determination of gases, liquids and substances. The method is based on the distribution of components between the two phases, the mobile and stationary phase. In gas chromatography, the mobile phase is a gas, called the carrier gas phase and is placed in a chromatographic column. The stationary phase in packed bed columns can be solid (activated carbon, silica, alumina, polymeric adsorbents, etc.). Each component of the sample through the column progresses on the distribution component and the equilibrium concentration of the component in old stationary and mobile phase occurs. Gradually deposited on the column in order of increasing values of distribution constants and enters the detector.[3] The mass spectrometer is very part of this experience place where takes place transfer lineconnected to GC ,ion trap assembly connected to vaccum assembly and electronic assemblies.The electronics assemblies then can be connected to personal computer or to computer interface.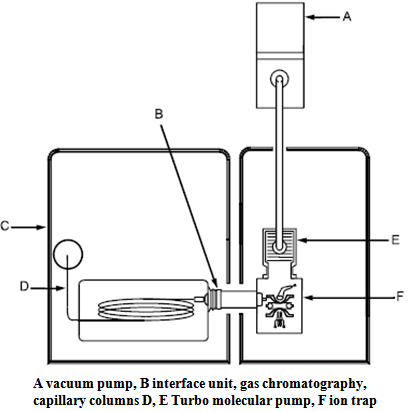 | Figure 1. Scheme GC- mass spectrum[3] |
The Saturn 2000 GC/MS has four principal components:- Gas chromatograph (GC)-Mass spectrometer (MS)- Data system (DS)- AutoSampler (optional)Table 1. Main patterns of gas chromatogram
 |
| |
|
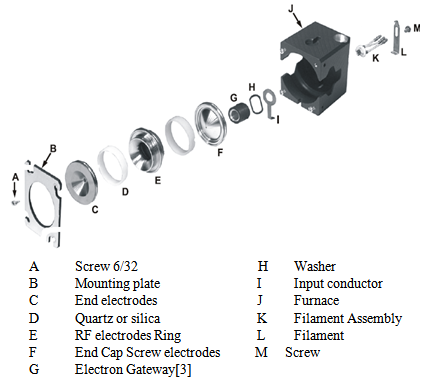 | Figure 2. Ion Trap [3] |
A short line-of-sight transfer line connects the GC and mass spectrometer. TheAutoSampler sits on top of the GC. A fused silica capillary column in the GC passes through the transfer line directlyinto the ion trap assembly. Samples are injected either manually or via the AutoSampler onto thecapillary column through the GC injection port.The gas chromatograph then separates the sample molecules. Effluent from the GC passes through the transfer line and into the ion trap. The sample moleculesnext undergo electron or chemical ionization before being analyzed according totheir mass-to-charge ratios.The ions are detected by an electron multiplier, which produces a signalproportional to the number of ions detected. The electron multiplier passes theion current signal to the system electronics, which in turn amplify the signal, digitize the result, and pass it on to the data system for further processing and display.Mass spectroscopy is one of the chemist’s tools in their arsenal. Through mass spectroscopy (MS), one can determine the molecular weight, molecular formula, and the functional groups present on a compound (Skoog 524). The molecular weight can be determined by identifying the molecular ion peak (Skoog 525). Looking at the mass to charge ratio (m/z) and the distribution of isotopes, one is able to determine an average mass for a compound. The mass contribution of each isotope is 2 determined by each isotope’s mass and relative abundance. Adding all of the isotopes’ mass contributions will then result in an average mass for the compound (Robinson 723). The molecular formula for a compound can then be ascertained from the relative peak heights of the isotopes (Skoog 525). Peak areas and heights can also be useful because they can indicate the concentration of various components present in the sample (Skoog 531-532). When looking at mass spectra, the majority of peaks that appear are attributed to fragments; these fragments are pieces of the original compound that have separated from the compound. The appearance of these fragments makes interpretation slightly more difficult, which is why the use of a library can become advantageous. By comparing your sample to knowns, a positive identification is possible (Skoog 524). So how does mass spectroscopy work? Upon passing through the column the particles continue on to the mass spectrometer where the sample is “bombarded by highenergy electrons” (Silberberg 54). These electrons collide with the atoms in the sample and, as a result, knock an electron off of the sample’s atoms, inducing a positively charged particle (Silberberg 54). The particles are then separated by a variety of means depending upon the type of mass spectrometer used. For example, in an ion trap mass spectrometer (which we use in our labs) the particles are deflected depending upon their mass/charge ratio (Skoog 517-518). A detector determines the particles’ “relative positions and abundances” which can then be used to determine the identity of the compound (Silberberg 54). The largest peak in the spectrum is called the base peak. The heights of all the additional peaks in the spectrum are then measured as a percent relative to the base peak (Skoog 499). The main advantage of interfacing a gas chromatograph with a mass spectrometer is its efficiency. With combined instrumentation, only one sample needs to be prepared. This saves on both time and materials. The physical bench space taken up is minimized as well. The GC/MS/MS allows one to obtain more detailed information about unknown compounds faster and more easily than the instruments separately. Combining these technologies allows one to analyze more complex compounds faster than a GC, MS, or GC/MS (Skoog 531).Ion trapTrap furnace generates heat for thermal control; it is unlikely that two fibers will have the same net inflow of electrons in the ion trap, so that the amplitude of the signal from two different fibers was the same.Electron gateElectron gate is a cylindrical electrode that controls the entry of electrons into the ion trap and its ion trap group has three stainless steel electrodes.Electron multiplierElectron multiplier is fixed in a preset position on the protective metal clip that can be easily removed and replaced.Stable readingFore line pump has two purposes. The pump is connected to the rear panel socket marked voltage - pump on the back of power supply side is outlet, which is controlled by a switch.Rear panelFore line pump used on the Saturn GC / MS is a two-stage rotary pump with a pumping speed of 90 ml/min and vacuum potential of 1.5 x 10-2 torr (2 x10-2 torr). RF generator assembly consists of a plate circuit detector link and shielded with coils under vacuum manifold housing, which surrounds the detector coil and the circuits.[20]Hard ionization technique is used in the gaseous phase. The most common and most advanced ionization method is well reproducible spectra production process. The interaction of the material with shock-accelerated electrons in high vacuum is preferred. Ionization of the source, transfer of substances to ionized state, the destruction of chemical bonds primarily resulting in ion formation and fragments. Analysis after separation in ion analyzer (separator) is distributed in space or time. A mixture of ions is produced in the ion source.DetectionThe detection is made from a direct stream of separate ions which is a signal proportional to the number of ions. It is transferred to a computer and processed using the software in the form of weight spectra.Registration and inspectionAlso, the computer registers the analytical data, manages and controls the operating conditions of the apparatus. Ionization is done in mass spectrum. Ionization of substances is essential for analysis as the information obtained applies only to charged particles or ion. Energy necessary for ionization depends on the substances. For experiments the following organic and conventional cottons were used :| Table 2. Samples used for analysis |
| | Reference cotton | Name | Origin | | Con-1 | Conventional cottonyear 2007 | Senegal | | Con-2 | Conventional cottonyear 2008 | Senegal | | Bio-1 | Organic cottonyear 2007 | Senegal | | Bio-2 | Organic cottonyear2008 | Senegal | | a-1 | Conventionalcotton Giza45-yoroc F6 AI/38KTM TUL | Egypt | | b-1 | Conventional cotton AI/69 KTM TUL | Russia | | c-1 | Conventional cotton T427, MVLSP AI/80KTM TUL | Indie |
|
|
8. Experimental Part
Results of gas chromatography with mass spectrum Varian 3800/2000Princip of methodSilica column (fused Silicium)Measurement parametersTemperature 80℃-180℃25℃ / min. Up to a temperature of 300℃Gas: Helium 1.3 ml / min.Split injector less than 2.0 ml, temperature 250℃Ionisation EI (electron ionisation)Dose per 1 ml of sample, solvent for rinsing 2 ml heptane.Based on samples of cotton available - cotton con - 1 of the 2007 harvest and cotton con-2 from the 2008 harvest, the cotton from Russia, China and Egypt, pesticide traces in the extract were observed.Preparation of extractSample of 1 g of cotton was extracted with 30 ml of dichloromethane (CH2Cl2) for 5 minutes to dissolve all anticipated substance in cotton sample. Using pesticides mix -10 (150 pesticides summary) the standard GC-MS or standard curve was determined with pesticide mix-10, but if the pesticides are not in the selected range (mix-10), they cannot be identified. The concentration of the extract was 0.03 g/ ml to 0.12 g / ml. Thus the occurrence of pesticides was observed on all cotton samples investigated. The analysis was done for the conventional cotton, organic cotton, conventional cotton with pesticide, organic with pesticide, a1-cotton from Egypt, b-1 from Russia and cotton c-1 of China. These extracts were monitored by GC-MS. The samples of organic cotton and conventional cotton were added with 1 ml mix-10.
 |
| |
|
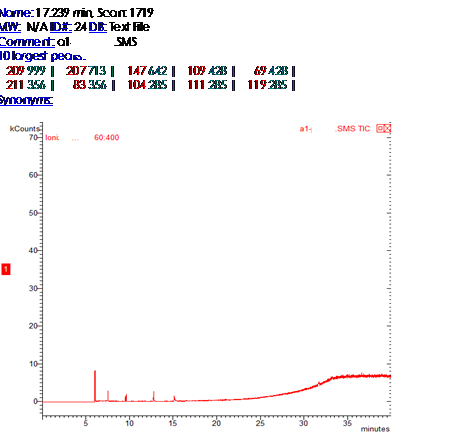
Chromatogram of 10 largest peak of cotton fiber Giza from Egypt 10 years in our laboratory
 |
| |
|
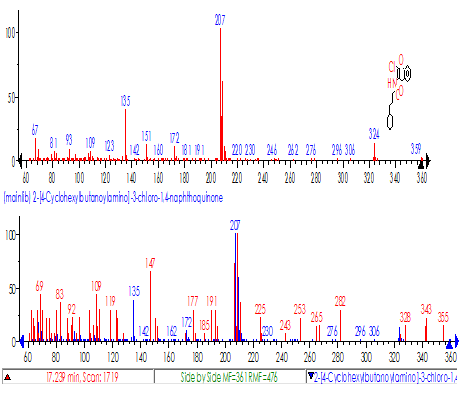
On axis x m/z (mass to charge)On y abundance ratioRelative abundance to mass-charge of cotton fiber Giza from Egypt 10 years in our laboratory,Which help to compare profile for two ion with so smilar ratio mass-charge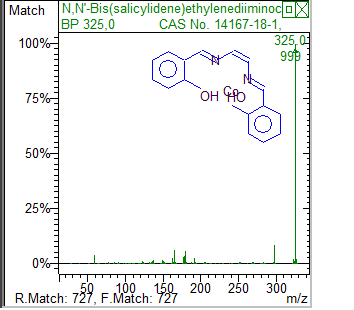 | Figure 3. Results from pesticides on cotton a-1 from Egypt from library(relative abundance to mass-charge) from library chemical formula and name of pesticide) |
 | Figure 4. Results of pesticides test on cotton b-1 from Russia |
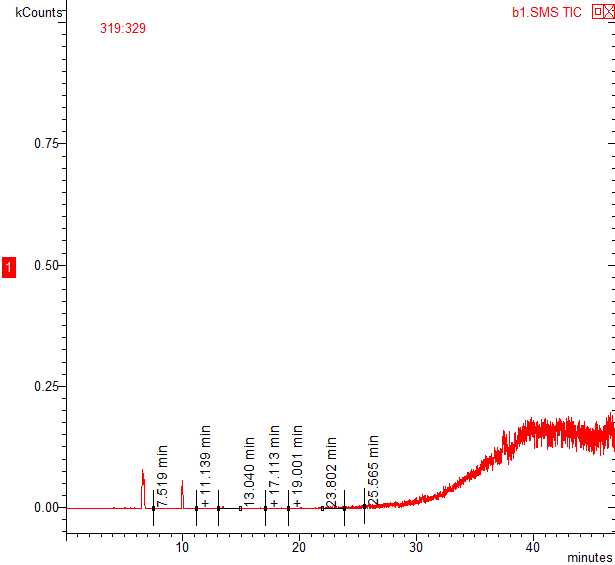
On axis x timeOn axis y intensity signal (kcount)Chromatogram of cotton fiber from Russia 10 years in our laboratory,with retention time(time elapsed from injection to peak max)
 |
| |
|
On axis x m/z(mass to charge)On y abundance ratioRelative abundance to mass-charge and results from library from India where you can read largest peak, chemical formula,name of pesticide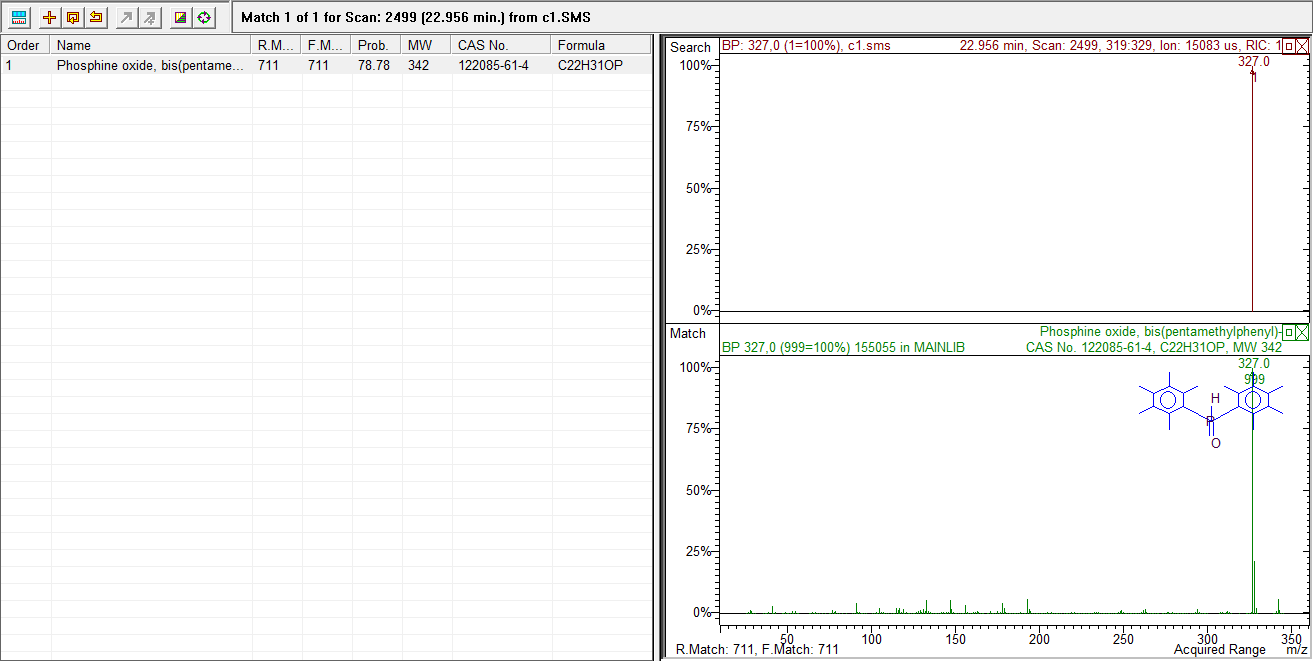 | Figure 5. Results of pesticides on cotton c- 1 from Indie from library |
Sample from Indie on left and the chemical formula of pesticide on right are well known pesticides in cotton cultivation, those sample of cotton are 10 years old in the laboratory of textile engineering.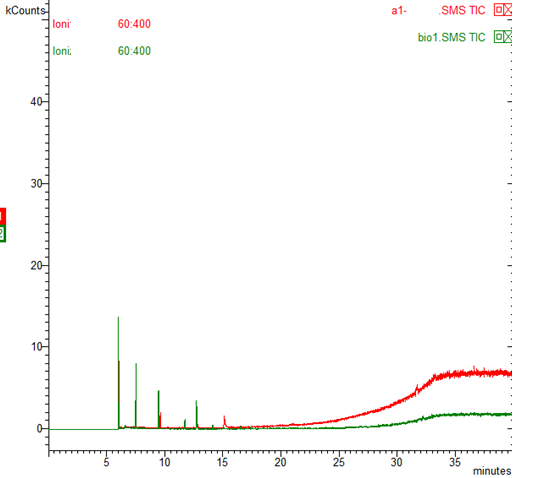 | Figure 6. Comparaison curve a-1 sample from Egypt and bio-1 organic cotton from |
The comparaisom between two chromatogram conventional fiber from Egypt and organic cotton bio-1 from Senegal show clearly the difference between organic and conventionalů according to this developed method.
9. Evaluation of Results, the Practical Lessons Learnt and General Conclusion for Practice
A methodology that allows detection of pesticides in cotton sample from Senegal and other countries of Russia, India and Egypt is proposed. The methodology is able to give the name of the chemical pesticides and formula of pesticide. Nowadays the properties of organic and conventional cotton are available. It is very difficult to distinguish biotechnology cotton, conventional cotton and organic cotton. Organic cotton is getting the benefits of the market in terms of trade as a whole, but conventional cotton price is determined by the class, which may affect. The proposed methodology could be applied to others naturals fibers, but of course it is necessary to study all properties of samples deeply in order to assess and finally to decide the suitable fiber. This methodology can be applied in others fields like toxicology, pharmaceutical or tracking drug on blood Marginal revenue may be interesting for farmers in case of organic cotton. Africa must be more competitive in terms of productivity and quality: compared to its competitors in the world market.
10. Conclusions and Discussion
Some argue that simply pesticides do not exist on cotton as confirmed by Institute in Bremen Germany. It is confirmed that it is impossible to detect pesticides on cotton, but the developed methodology detected pesticide in cotton fiber; it gives the chemical of pesticide and the name of the pesticide. Based on their tests on samples of cotton from Senegal, but under the proposed detection methodology that was developed in department of mechatronics, Institute of nanotechnology, and Applied Informatics, the chemical pesticides can be detected. The pesticides tests were conducted also with the old cotton samples (15 years) of cotton (Syria, Egypt, and China). The methodology developed in this text, today confirms that this method can detect pesticide for cotton.Based on this work, one can detect traces of pesticides in conventional and organic cotton through the proposed method using the apparatus GC-MS gas chromatography with mass spectrum variant 3800/2000, based on the principle that is proposed in this work. It confirmed the presence of pesticides that were used in Senegal (con-1, con-2), in Egypt ( a-1), in Russia ( b-1), in China ( c-1). That is why it is recommended for the certification of organic cotton and all natural fibers from plants, gives better resolution of the presence of pesticides in cotton fiber. In terms of the properties, the organic and conventional cotton is not very different. This method to detect pesticides in cotton samples, which was an intention, to distinguish between organic cotton and conventional cotton is acceptable. Importance of promoting organic plants underlines issues and characteristics of organic cotton and conventional cotton and how to determine pesticides in cotton fiber and thus certified as organic cotton and naturals plants. I hope my research gonna help for better understanding the detection of pesticide on cotton fiber and all naturals fibers.
References
| [1] | Brushwood, D.E. and Perkins, H.H., Jr., Text. Chem. Color., 26, 32, 2006 |
| [2] | Diop.B Journal renaissance cotonniere, Sodefitex Dakar Senegal No 1, 2008 |
| [3] | Varian, Inc. Printed in U.S.A. 03-914978-00:Rev.42004-2007 |
| [4] | Brushwood, D.E. and Perkins, H.H., Jr., Text. Chem. Color., 26, 32, 2006 |
| [5] | Hebert A.: Le coton fil des temps et des cultures brochure SIA, CIRAD, CIRAD p.2, 02/2006, www.cirad.fr |
| [6] | Diop.B Journal renaissance cotonniere, Sodefitex Dakar Senegal No 5, 2008 |
| [7] | Note cotonniere dagris-geocoton janvier fevrier-2008 |
| [8] | Phillip J. Wakelyn, Noelie R. Bertoniere, Alfred Dexter, French Devron Cotton fibers chemistry and technology third edition, Taylor and Francis 2007 International Fiber Science and Technology |
| [9] | International market for organic cotton and eco textile August 2002 |
| [10] | HLUČEK, J. Livestock manure. Mendel University of Agriculture and Forestry in Brno: Multitexty[online]. 2004 |
| [11] | Facts and figures: World cotton history. Cotton Australia [online].[cit. 04/03/2009]. |
| [12] | http://www.qmagazin.cz/textil-a-obuv/strucny-pruvodce-biotextilem-biobavlna.html Date 11.07.2009 |
| [13] | Henry JB. Clinical diagnosis and management by laboratory methods. 20th ed. Philadelphia: WB Saunders; 2,001th At U.S. Department of Health and Human Services. Clinical Laboratory Improvement Amendments Feb 28; 57 (40) : 7002-288th , Fed Registration 1992 |
| [14] | Kraft J. Guidelines for laboratory exercises in Analytical Chemistry. Institute of Chemical Technology, Prague 2001, |
| [15] | Varian, Inc. Printed in U.S.A. 03-914978-00:Rev.42004-2007 |
| [16] | Machaňová, D. Munchausen, M.: Environmental aspects of textile processes. Text book TU Liberec, 2005 |
| [17] | Kovacic V.- Militký J. –Rubnerová J. International scientific - technical conference Modernization of teaching in technically oriented fields and subjects Faculty of Education, Palacky University Olomouc, 25 and 26 6th 1997 |
| [18] | Pavel. Assoc. The Czech Agricultural University in Prague 2007 May |
| [19] | Brushwood, D.E. and Perkins, H.H., Jr., Text. Chem. Color., 26, 32, 2006 |
| [20] | http://www.velkaepocha.sk/2010050213159/Organicka-bavlna.html 14.1.2012 |
| [21] | M. Solnicková BC work, TUL LIBEREC 2009 , |
| [22] | http://www.uv.es/~pico/papers/35.pdf date 01/12/2012trends in analytical chemistry vol 23, no 10-11 2004 |
| [23] | Lothar and Hoffman Detection of genetically modified cotton GMBH Bremen Germany 2008 |







 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML


