-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Textile Science
p-ISSN: 2325-0119 e-ISSN: 2325-0100
2013; 2(2): 30-35
doi:10.5923/j.textile.20130202.03
Dyeing of Cationized Cotton Fabrics with Natural Dye Extracted from Acacia
Saleh M S1, Abd El-Baset Y A1, Kh. El-Badry2
1Cotton Research Institute, Textile Chemistry Department, Giza, Egypt
2Faculty of Education, Industrial Education Department, Helwan University, Cairo, Egypt
Correspondence to: Saleh M S, Cotton Research Institute, Textile Chemistry Department, Giza, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Cotton, like most textile fibres, was negative charged in neutral and alkali aqueous solutions. Dyestuffs, optical brighteners and finishing agents in aqueous solution have the same electrical charge as cotton. Therefore, adsorption processes were difficult due to these repulsive forces. This paper studied the dyeing of alkaline extraction of acacia bark after the finishing of modified cotton – by cationization. In this paper, cationization of cotton fabric for achieving electropositive charge and better adsorption properties was carried out with cationic compound produced from extractable solution of chicken feather. The dyeing of cationized cotton fabrics using colouring matter extracted from acacia bark has been studied. The application of the extractable solution of chicken feather with cotton was studied. Color measurements were carried out to evaluate the shade obtained. The dyed fabrics were subjected for analysis in terms of K/S, CIE L* a* b*, hue and chroma as well as their fastness properties. The results indicated the dye extraction was more effective at alkaline medium. The colour measurement values were found to be better with cationization than the untreated samples. The results with the fastness properties were from fair for untreated samples to good with the cationized fabrics.
Keywords: KeywordsAcacia Bark, Chicken Feather, Cationization, Color Strength
Cite this paper: Saleh M S, Abd El-Baset Y A, Kh. El-Badry, Dyeing of Cationized Cotton Fabrics with Natural Dye Extracted from Acacia, International Journal of Textile Science, Vol. 2 No. 2, 2013, pp. 30-35. doi: 10.5923/j.textile.20130202.03.
Article Outline
1. Introduction
- Specific adsorption of ions or dissociation of the surface groups in aqueous solution results with their surface charge. Fiber surface charge depends upon their chemical and physical-chemical structure, swelling capacity as well as upon ionogenity, structure and concentration of adsorbate. Different treatments, mostly alkaline modifications of fibers, change surface charge and adsorption ability. Adsorption properties of fibers are influenced by change of surface charge[1]. At the interface of an electrically charged textile fibers and an aqueous solution of electrolyte, surfactants or dyes set up an electric double layer. Moving one of these two charged surfaces results by electrokinetic (zeta) potential. Zeta potential is not material constant, but gives information about nature and dissociation of functional groups, hydrophilicity or hydrophobicity of fiber surface as well as ion or water sorption. This potential plays an important role in the electrical characterization of textiles in wet processing,[2].Egypt has an agricultural tradition which goes back thousands of years, and plans to expand this tradition in the future. In order to combine the old tradition with modern technologies to achieve sustainable development, waste should be treated as a by-product. A renewed international interest has arisen in natural dyes due to increased awareness of the environmental and health hazards associated with the synthesis, processing and use of synthetic dyes.Textile processing industry is one of the major environmental polluters. In order to process a ton of textile, one might have to use as much as 230 to 270 tons of water. The effluent generated by this much water would pollute the environment as it contains a heavy load of chemicals including dyes used during textile processing,[3]. There are two main ways to limit the environmental impact of textile processing. One is to construct sufficiently large and highly effective effluent treatment plants, and the other way is to make use of dyes and chemicals that are environment friendly.Natural dyes are known for their use in colouring of food substrate, leather, wood as well as natural fibers like wool, silk, cotton and flax as major areas of application since ancient times. Natural dyes may have a wide range of shades, and can be obtained from various parts of plants including roots, bark, leaves, flowers, and fruit[4].The use of non-toxic and eco-friendly natural dyes on textiles has become a matter of significant importance because of the increased environmental awareness in order to avoid some hazardous synthetic dyes. However, worldwide use of natural dyes for the coloration of textiles has mainly been confined to craftsman, small scale dyers and printers as well as small scale exporters and producers dealing with high valued eco-friendly textile production and sales[5].Acacia is a genus of shrubs and trees of Mimosoideae family. The local name of acacia is Nile or scented-pod acacia, Gum Arabic tree, Egyptian thorn. In Egypt the plant is used as a medication for expelling worms (vermifuge), treat diarrhea, skin diseases and acts as an astringent drug agent from internal bleeding. Phloem strands from its peeled bark are folded into a ball and juice from chewing it is swallowed for relief from sore throats and coughs. A beverage made from boiling its leaves is drunk for relief from chest pains and for pneumonia treatment. A decoction made from boiling its roots is drunk as a treatment for indigestion or stomach troubles. Its roots are taken to treat gonorrhea. A decoction made from its bark is given to children to aid in digestion and is also used as a mouth rinse for a period of about three or four days for both males and females respectively for relief from tooth aches. A decoction made from its roots is taken as a treatment for impotence.A mixture made from mixing its fresh fruit together with its bark is stored for a period of about four to six months and rubbed sparingly onto soaring gums or onto a painful tooth for relief. A mixture made from mixing its dried fruit together with the bark of the tree species Mangifera indica L. (Mango) and that of the plant species Zinm are pounded, strained into a powder form and put onto a decayed tooth cavity for removal or it may be rubbed onto soaring gums for relief. A decoction made from both its fruits and its leaves is used as a mouthwash for relief from gingivitis. The plant when combined together with the plant species Entada Africana and Mimosa pigra L. are used for treating diarrhea, genitourinary infections and for relief from typhoid fevers. Its stem bark extracts exhibit antibacterial properties that act against the bacteria Salmonella typhi (typhoid). A decoction made from its bark is prepared with the leaves of the plant species Pseudocedrela kotschyi (Schweinf.) harms for relief from tooth aches.Its bark is used as a broad spectrum herbal remedy for livestock. A decoction made from its bark is drunk to act as an aphrodisiac or as a powerful sexual stimulant,[6]. It is used for making cosmetic products such as tonic lotions. Its bark is used for dyeing and is rich in phenolic constituents such as Tannins, quercetin, catechin, gallic acid, epigallocatechin and acacetin[7].Reference[8], pointed out the application by padding cotton fabric with 10 g/l of two natural dyes derived from the Acacia plant family.Cotton dyeing with natural dye extracted from pomegranate (Punica granatum) peel was studied by Kulkarni et al. Kumaresan et al pointed out the application of eco-friendly natural dye on cotton using combination of mordant's. Reference[9], described ethanolic extract of Sesbania Aculeata as natural dye for Green Dyeing of Cotton. The utilization or recycling of chicken feather waste to a useful material as a cleaner product in the industrial processes has proceeded in the recent years. Poultry chicken feathers represent about 6.0% of the total weight of mature chicken lead to environmental problems as waste – by product at commercially poultry plants[10].Reference[11] showed that approximately two or four billion pounds of poultry feathers as a natural source of active amino acids are produced every year by the poultry producing industry. Treating textile materials with selected amines from natural resources as cheaper and environmental friendly chemicals provide the aesthetics and/or make the materials more respective to dyes, and more UV-protective for the human skin have been used recently. The treatment causes cellulosic material to become more cationic and thus more respective to anionic dyes without stiffening,[12]. Our previous work described the utilization of chicken feather waste to improve the properties of fabrics made of some Egyptian cotton varieties[13].
2. Materials and Methods
2.1. Materials
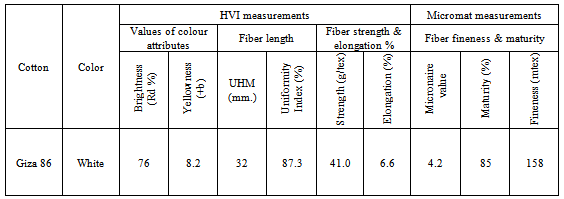
- Bleached cotton fabrics with the same structure of long staple Egyptian cotton variety Giza 86 were purchased from El-Nasr for weaving and spinning Company- El-Mehala, Egypt, and used throughout this study. Giza 86 was measured in Cotton Research Institute (CRI) labs by HVI spectrum instrument. The fiber properties of this cotton material were presented in table (1).
|
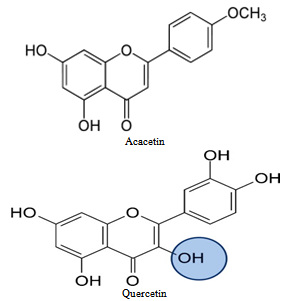 | Figure 1. The structural formula of main colouring components present in acacia bark |
2.2. Methods
2.2.1. Synthesis of Cationizing Agent from Chicken Feather (CF)
- Freshly plucked wet feathers were cleaned with water at 60℃ followed by water at room temperature. Wet feather were dried in a ventilated oven (Memmert-UL500- Italy) at 40℃ for 72 hrs. The feather was cut to about 2 mm in length. 50 g of these materials were treated in a Soxhlet device for 12 hrs with petroleum ether (boiling range 40-60℃) to remove grease. The petroleum ether was evaporated and the dry feathers were stored at room temperature under closed conditions.
2.2.2. Treatment with Alkaline Solutions[NaOH]
- 10g of CF were stirred at 80 rpm with 20 ml 0.95N NaOH at 70℃ for one hour. The produced solutions were filtered to remove any ash and wax residues, and neutralized with aqueous solution containing 0.1% acetic acid, followed by a stream of hot water (80-85℃) washing to ensure removal of residual chemicals. Samples were dried in an oven at 100℃ for 60 minutes.
2.2.3. Cationization Pretreatment
- Pretreatment was carried out according to our previous work[13]. Fabric was two-dipped/ two-nipped in the bath with a wet pickup of 70 to 80%. After padding, it was dried at 80-85℃ for 15 minutes and cured at temperature of 130℃ for 3 minutes.
2.2.4. Extraction and Dyeing Condition
- Extraction of the Acacia bark dye was carried out according to the method explained by reference[14]. The optimum condition for the dye extracted was as the following: Extraction Medium 0.30M NaOH, Temperature 100℃, Time 75minutes, and L:R 1:12.5. the exhaustion dyeing process of the cationized cotton fabric was carried out using IR dyeing machine at temperature 70℃, Time 100 minutes, and L:R 1:15. The dyed samples were washed with hot water at 80℃, then washed with cold water and finally dried in oven at 90℃.
2.3. Measurements
2.3.1. Color Measurement
- Several colour measurements were carried out to evaluate the shades obtained on dyeing cotton with alkaline extract of Acacia. The colour strength (K/S value) was assessed using the Kubelka – Munk equation[15]. K / S = (1- R )2 / 2RWhere R is the decimal fraction of the reflectance of dyed fabric.The CIE L*a*b*, values were measured, Chroma (C) is a measure of intensity or saturation of colour and Hue angle (H) is derived from the two coordinates a* and b*.
2.3.2. Fastness Testing
- After the colour measurements, the dyed samples were tested for fastness to various agencies:
2.3.2.1. Wash Fastness
- Wash fastness of the dyed samples was analyzed as per the AATCC (American Association of Textile Chemists and Colourists) Test Method 61, 2 (A). The dyed specimens were cut in size in 5 x 10 cm. and were washed in Launder-o-meter. A detergent solution was prepared using detergent at concentration of 5 g/l. The prepared samples were then placed in the canisters along with the 8-10 steel balls and then fixed in the launder-o-meter. These were subject to 5 cycles for approx. 20 minutes with temperature maintained at 38℃.
2.3.2.2. Rubbing Fastness
- Dry and wet rubbing fastness was tested as per AATCC Test Method 8. For testing the dry specimen, the dyed sample was fastened to crock meter base and rubbed with white test cloth under controlled conditions for approximately 20 cycles. Similarly, for testing the wet rubbing fastness, test samples were dipped in distilled water and were squeezed between blotting papers less than 454 grams (l lb. or one second, and then the entire process of dry rubbing fastness was repeated.
2.3.2.3. Sun-light Fastness
- Sun-light fastness was evaluated with AATCC Test method 16 specifications in coordination with AATCC Test method181. The dyed samples were tested in day light behind glass.
3. Results and Discussion
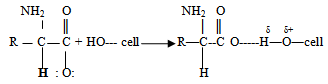
- Cationization of CF on cotton fabrics is due to the H- bonding formed between free hydroxyl groups of the cellulose with carboxylate anion of the amino acid as shown in the following equation:
 The complex formation between the amide groups of the CF Present on the cotton surface with the hydroxyl group of the extract dye from the acacia bark was shown in the following diagram:
The complex formation between the amide groups of the CF Present on the cotton surface with the hydroxyl group of the extract dye from the acacia bark was shown in the following diagram: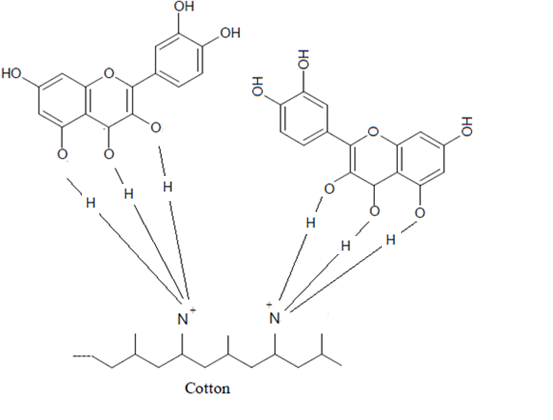 Figure 2, illustrates the effect on relative colour strength values of dyed cotton with aqueous extracts. As shown, more than double amount of relative colour strength (%) on cationized cotton fabric was obtained.
Figure 2, illustrates the effect on relative colour strength values of dyed cotton with aqueous extracts. As shown, more than double amount of relative colour strength (%) on cationized cotton fabric was obtained.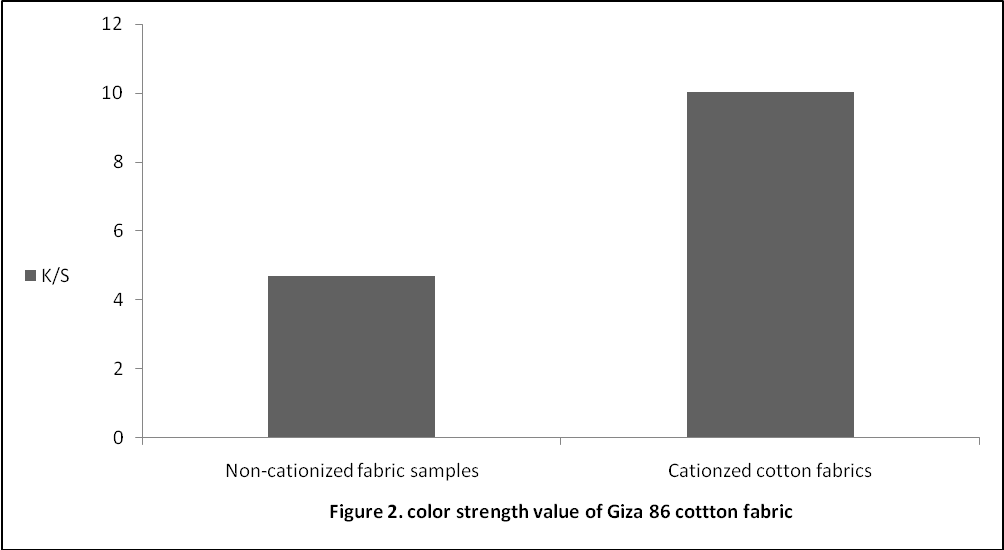 | Figure 2. Colour strength value of Giza 86 cotton fabric |
 The effect of colour coordinates was shown in Figure 3. Since hue (h) is little affected by the extraction medium as hue values are 54.55 & 55.07 for non-cationized and cationzed cotton fabric respectively. The lightness L* values are attributed with relative colour strength. As higher the relative colour strength value lowers its L* value. This could be attributed with more extraction of colouring component in 0.3M NaOH due to the presence of acidic hydroxyl groups in colouring component (Polyphenolics and Flavonoids). Both samples have reddish and yellowish tone with positive a* & b* values. The sample dyed with colouring component extracted in the 0.3M NaOH was less yellower with b* 10.56 value for the non-cationized cotton fabrics in comparison with b* 15.71 for cationized cotton sample. Furthermore both have fairly good chromaticity (C*) values but more dullness was observed in catonized dyed sample. The fastness properties of the fabric dyed without and with cationization is indicated in Table 2. The result being assessed in the usual way in term of the grey scale values for the staining of adjacent cotton material. The fastness properties of the fabric dyed without and with cationization is indicated in Table 2. The result being assessed in the usual way in term of the grey scale values for the staining of adjacent cotton material and change in shade as well. The results indicated that the use of CF as cationizing agent was advantageous for the wash fastness in which shows 4 grey scales rating in comparison to sample dyed without cationization. Thus cationization treatment was proved to be better with reference to wash fastness. This could be attributed to the formation of insoluble colour complex on fabric. The higher grey scale rating could also be attributed with coordination complexes.Light fastness values of fabric dyed with Acacia’s bark extract with and without cationization are indicated in Table. 2. The result indicated that the use of CF was advantageous for cotton fabric dyed with Acacia bark extract. The light fastness of dyed fabric increase with increasing of dye concentration, the main cause being an increase in average size of submicroscopic particles in which the dyes form in the fiber because the smaller area of dyes exposed to air and light (Cristea and Vilarem, 2006). Cationization not only increase the dye uptake for fabric, i.e. dye concentration into fabric but also from the metal dye complex, which are mostly more stable than colouring component itself. Although all samples dyed in presence of CF attained better light fastness than sample dyed without cationization. It was confirmed by strong colour changing more fastness to washing and less fading on light exposure, cationization form metal dye complexes which are able to form coordination linkages between the metal atom and cotton fabric,[17]. cationization samples had shown better rubbing fastness properties in comparison to sample dyed without cationization due to the increased of dye uptake in case of i.e. depth of shade was improved. Secondly, cationization forms an insoluble colour complex which was insoluble and remained as aggregate of molecules on fabric surface.
The effect of colour coordinates was shown in Figure 3. Since hue (h) is little affected by the extraction medium as hue values are 54.55 & 55.07 for non-cationized and cationzed cotton fabric respectively. The lightness L* values are attributed with relative colour strength. As higher the relative colour strength value lowers its L* value. This could be attributed with more extraction of colouring component in 0.3M NaOH due to the presence of acidic hydroxyl groups in colouring component (Polyphenolics and Flavonoids). Both samples have reddish and yellowish tone with positive a* & b* values. The sample dyed with colouring component extracted in the 0.3M NaOH was less yellower with b* 10.56 value for the non-cationized cotton fabrics in comparison with b* 15.71 for cationized cotton sample. Furthermore both have fairly good chromaticity (C*) values but more dullness was observed in catonized dyed sample. The fastness properties of the fabric dyed without and with cationization is indicated in Table 2. The result being assessed in the usual way in term of the grey scale values for the staining of adjacent cotton material. The fastness properties of the fabric dyed without and with cationization is indicated in Table 2. The result being assessed in the usual way in term of the grey scale values for the staining of adjacent cotton material and change in shade as well. The results indicated that the use of CF as cationizing agent was advantageous for the wash fastness in which shows 4 grey scales rating in comparison to sample dyed without cationization. Thus cationization treatment was proved to be better with reference to wash fastness. This could be attributed to the formation of insoluble colour complex on fabric. The higher grey scale rating could also be attributed with coordination complexes.Light fastness values of fabric dyed with Acacia’s bark extract with and without cationization are indicated in Table. 2. The result indicated that the use of CF was advantageous for cotton fabric dyed with Acacia bark extract. The light fastness of dyed fabric increase with increasing of dye concentration, the main cause being an increase in average size of submicroscopic particles in which the dyes form in the fiber because the smaller area of dyes exposed to air and light (Cristea and Vilarem, 2006). Cationization not only increase the dye uptake for fabric, i.e. dye concentration into fabric but also from the metal dye complex, which are mostly more stable than colouring component itself. Although all samples dyed in presence of CF attained better light fastness than sample dyed without cationization. It was confirmed by strong colour changing more fastness to washing and less fading on light exposure, cationization form metal dye complexes which are able to form coordination linkages between the metal atom and cotton fabric,[17]. cationization samples had shown better rubbing fastness properties in comparison to sample dyed without cationization due to the increased of dye uptake in case of i.e. depth of shade was improved. Secondly, cationization forms an insoluble colour complex which was insoluble and remained as aggregate of molecules on fabric surface. 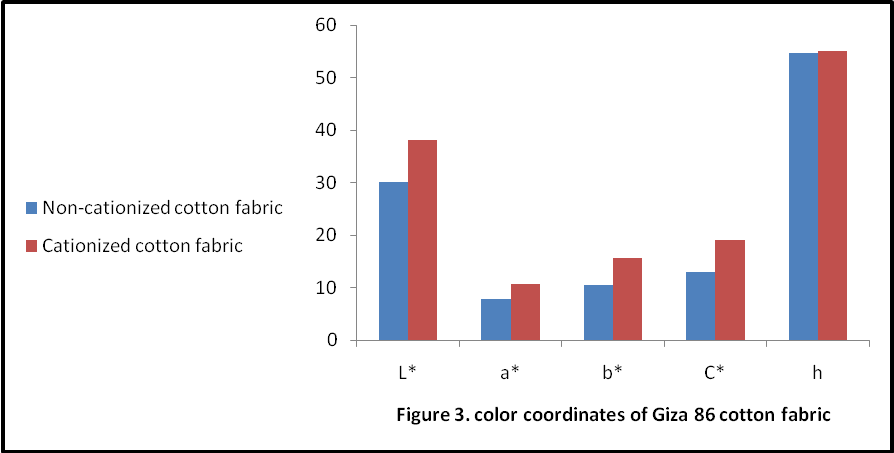 | Figure 3. Colour coordinates of Giza 86 cotton fabric |
|
4. Conclusions
- Cationization, as modification processes of cotton fabrics, caused the change of its ionic character resulting in different zeta potential, surface charge and change of its ionic character resulting in different zeta potential, surface charge and plant water extract adsorption. By cationaization, an electrical charge of cotton fabrics was noticeable changed, from negative to positive values. Electropositive cotton shows better adsorption of anionic substances because the adsorption occurs mainly by electrostatic interactions between charged particles and specific accessible cotton fiber groups. Based on all achieved results, it is evident that alkaline extract of acacia bark shows marigold anionic character. Therefore, from the environmental point of view it is a good substitution of chemical dyestuffs with plant extracts, considering the ionic character of both, fabric and plant extract solution. That is providing not only a strategy for reducing risks and pollutants, but also an opportunity for new markets and new businesses that could be developed implementing environment to market policy.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML