-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Textile Science
p-ISSN: 2325-0119 e-ISSN: 2325-0100
2013; 2(2): 26-29
doi:10.5923/j.textile.20130202.02
Reaction Mechanism of Single Step Fixation Process of Reactive Printing and Crease Resistance Finishing of Cotton Fabrics
FarehaAsim1, Naheed Kausar2, Muzzaffar Mahmood3
1Textile Engineering Department, NED University of Engineering & Technology Karachi, Pakistan
2Research Industrialization Division, PCSIR Laboratories Complex Karachi, Pakistan
3Mechanical Engineering Department, NED University of Engineering & Technology Karachi, Pakistan
Correspondence to: FarehaAsim, Textile Engineering Department, NED University of Engineering & Technology Karachi, Pakistan.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The mechanism of dye fixation on cotton fabric using single step fixation of reactive printing and crease resistance finishing has been examined in this research work. Monochlorotriazine based reactive dyes can chemically combine with hydroxyl groups of cellulose fabric by a nucleophilic substitution reaction and as a result an ether linkage is formed. In contrast, modified DMDHEU mainly contains N-alkoxymethyl groups and during the finishing process, the most preferable reaction of N-methylol anions is with hydroxyl groups of the cellulose. The developed reaction mechanism indicates that MCT based reactive dyes can be chemically combined with hydroxyl groups of cellulose as well as modified DMDHEU that is cross linked with cellulose fabric in a single step fixation of reactive printing and crease resistance finishing.
Keywords: Crease Resistance Finishing, DMDHEU, MCT, Reactive Printing
Cite this paper: FarehaAsim, Naheed Kausar, Muzzaffar Mahmood, Reaction Mechanism of Single Step Fixation Process of Reactive Printing and Crease Resistance Finishing of Cotton Fabrics, International Journal of Textile Science, Vol. 2 No. 2, 2013, pp. 26-29. doi: 10.5923/j.textile.20130202.02.
Article Outline
1. Introduction
- The theoretical fixation mechanism of Monochlorotriazine based reactive (MCT) dye and modified dimethyloldihydroxy ethylene urea (modified DMDHEU) using single step fixation process is being discussed in this research work.Reactive dyes are the most popular application class used to dye cellulose fibres. It forms covalent bond with cellulose. Due to the strong primary bonding between dyes and fibres, it possesses high level of fastness. The major drawback of reactive dyes is hydrolysis in which dye instead of reacting with fibre reacts with the hydroxide anion of the base[1].Cotton is an important fibre in textiles, because of its numerous advantages. One of the main disadvantages of cotton is creasing after washing[2]. On creasing the cotton fabrics, the molecular chains in the amorphous region slip past each other, breaking the weak hydrogen bonds. The stretched chains then form hydrogen bonds in the stretched places and thus the fabric holds the creases. The mechanism of the crease recovery process in cotton fibres is based on introducing stable cross-links so as to prevent slippage of molecular chains[3].Different types of formaldehyde based crease resistants have been used for crease resistance finishing of cotton fabric. Dimethyloldihydroxy ethylene urea (DMDHEU) based crease resistants are commonly used in the textile industry due to their numerous advantageous[4]. In the present research work, modified DMDHEU has been used which has low formaldehyde content unlike unmodified DMDHEU[4]. As discussed, MCT based reactive dye and modified DMDHEU can react with the hydroxyl groups of cotton fabric. The rate of bond formation between substrate and crease resistant finish or MCT dye is not only influenced by various external parameters but it is also greatly dependent on the nature of the alkyl group attached with modified DMDHEU and various other functional groups attached with the MCT dye.Various attempts[5-10] have been made on combining crease resistance finishing with reactive dyeing and finishing process to capture the potential time, energy, and other savings associated with the single step process. Some research[11-14] was found regarding a single step process of reactive printing and crease resistance finishing. However, there were no data reported regarding the reaction mechanism of the reactive dye and the crease resistant in the single step print-finish fixation of cotton fabrics.
2. Single Step Fixation Process
2.1. Preparation of Print Paste
- A concentration of 3% w/w of Alginate thickener was added to produce stock paste, with continuous high speed stirring, to the required volume of water. This was followed by the gradual addition of alkali: sodium bicarbonate 30 g/kg, reduction inhibitor: Revatol S 50 gm/kg and sequestrant: sodium hexameta phosphate 5 gm/kg with continuous stirring giving a final stock paste viscosity of 60-65dPa. The 3% Drimarine Red P2B(Clariant) based on MCT reactive group is finally added to the stock paste.
2.2. Preparation of Crease Resistance Finish Liquor
- The crease resistant finishing liquor was prepared by using modified dimethylol di hydroxyl ethylene urea: Fixapert F-ECO (BASF) 100 g/l, silicon softener:Solusoft MW 20g/l, Ionic softener: Ceranine-L 20g/l, wetting agent: Invadine PBN 5g/l and Catalyst: MgCl215 g/l.
3. Application Method
- The single step fixation process is carried out as follows;In the first step the fabric was immersed in an aqueous solution of crease resistant finish liquor containing, and then squeezed to obtain a 70% wet pickup. The wet fabric was then dried at 100°C for 1 min. In the second stage the treated fabric was printed by the lab scale Rotary Printing machine (Zimmer).The print-finish fabric is simultaneously fixed through the Econtrol process at 130-135˚C for 5 minutes The fixed samples are finally washed in 1g/l non-ionic detergent until all unreacted dyes and chemicals were removed from the fabric surface.
4. Reaction Mechanism
- Modified DMDHEU mainly contains N-alkoxymethyl groups (Fig. 1). During the finishing process, the most preferable reaction of N-methylol anions is with hydroxyl groups of the cellulose (Eq. 1).
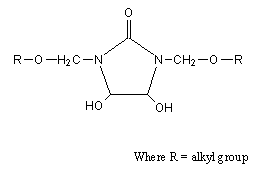 | Figure 1. General structure of modified DMDHEU |
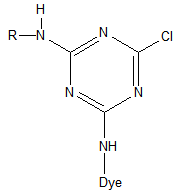 | Figure 2. General structure of MCT reactive dye |
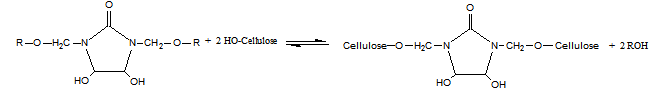 | (1) |
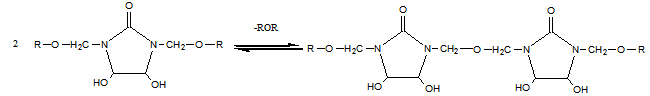 | (2) |
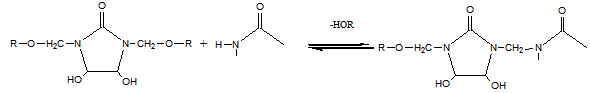 | (3) |
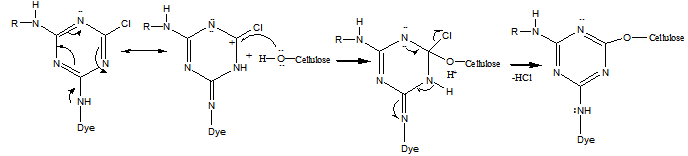 | (4) |
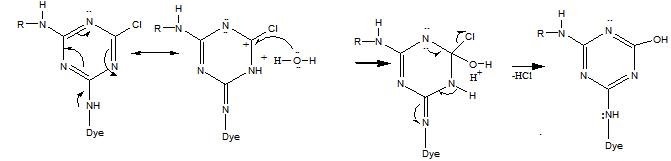 | (5) |
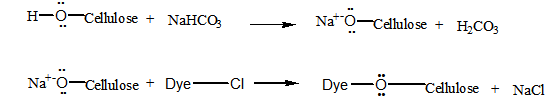 | (6,7) |
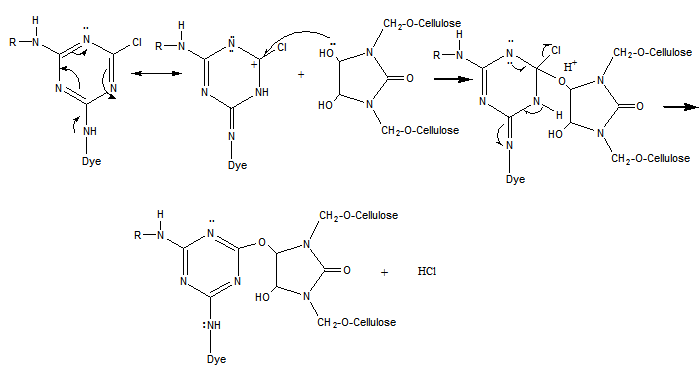 | (8) |
5. Conclusions
- A reaction mechanism for the single step process with a Monochlorotriazine based reactive dye and modified dimethyloldihydroxy ethylene urea based crease resistant has been developed validating the premise that the reactive dyes do chemically combine with hydroxyl groups of the cotton fabric as well as a crease resistant that is cross linked with the cotton fabric in the single step fixation process under certain reaction conditions.
ACKNOWLEDGEMENTS
- The authors acknowledge the permission given by Gul Ahmed Textile Mills Ltd, for carrying out the necessary experimental work and PCSIR for their assistance.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML