-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Textile Science
p-ISSN: 2325-0119 e-ISSN: 2325-0100
2013; 2(1): 1-6
doi:10.5923/j.textile.20130201.01
Characterization of Dyeing P/C Blends Fabric: a Thermodynamic View
Kunal Singha
Department of Textile Technology, Panipat Institute of Engineering & Technology, Harayana, India
Correspondence to: Kunal Singha, Department of Textile Technology, Panipat Institute of Engineering & Technology, Harayana, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The dyeing of the polyester and cotton blend fabric (P/C fabric or PCF) has becomes a challenge to the modern textile industries due to its variation in color value, shed depth, tensile strength and surface residual weight loss. All of this drawback can be control by the proper selection of the dye-fiber combination which ultimately governs the XRD, SEM, FTIR analysis of the dyed blend fabric. The water fastness or wash fastness and light fastness can be also improved by the proper analysis of the thermodynamic equilibrium of dye fiber bond and its stoichiometry.
Keywords: Polyester , Cotton Blend Fabric (P/C Fabric Or PCF), Color Value, Shed Depth, Tensile Strength, Surface Residual Weight Loss, XRD, SEM, FTIR, Thermodynamic Equilibrium
Cite this paper: Kunal Singha, Characterization of Dyeing P/C Blends Fabric: a Thermodynamic View, International Journal of Textile Science, Vol. 2 No. 1, 2013, pp. 1-6. doi: 10.5923/j.textile.20130201.01.
Article Outline
1. Introduction
- Dyeing is the process of adding color to textile products like fibers, yarns, and fabrics. Dyeing is normally done in a special solution containing dyes and particular chemical material. Dyeing is the process of imparting colors to a textile material through a dye (color). Dyes are obtained from flowers, nuts, berries and other forms of vegetables and plants as well as from animal and mineral sources. These are known as natural dyes. The other class of dyes is known as synthetic dyes. These are based on a particular type of chemical compositions. Some of these dyes are- Acid (Anionic) dyes, Basic (Cationic) dyes, neutral-premetalized dyes, sulfur dyes, vat dyes, reactive dyes, pigment dyes[3]. Color is applied to fabric by different methods of dyeing for different types of fiber and at different stages of the textile production process. These methods include direct dyeing; Stock dyeing; top dyeing; Yarn dyeing; Piece dyeing; Solution is pigmenting or dope dyeing[2,4], garment dyeing etc.
1.1. Disperse dye
- Disperse dyes give excellent overall fastness properties with polyester where it gives very poor fastness properties with cellulose. The reason behind this is disperse dyes does not have any substantively & affinity towards cotton & cotton cannot be dyed at very high temperature as like Polyester. Hence P/C blend dyeing will not give sufficient fastness properties compared to 100% Polyester[5]. Normally in industries a recipe used for dyeing 100% Polyester is also applied for different proportions of P/C blends and then it is topped up with reactive dyes to match the shade but it will not give sufficient fastness properties[6]. 100% PET means for strength, crease resistance but it cannot be wearable because of low moisture content, static generation and accumulation, so P/C blend enters in market because it has a advantages of both PET and cellulose and can be wearable[7]. PET cotton blend has got lot of advantages from user point of view but from dyers point of view it was difficult to dye the blends. P/C blends does not give sufficient fastness properties as like 100% PET with disperse dyes. In industries, single recipe used for 100% PET is also applied for different proportions of P/C blends& then it was topped up with reactive dyes to match the standard shade but the fastness properties of these blend is a big question mark. While using disperse dyes (Fig 2) for dyeing P/C blends[8], it is noticed that the PET portion is not only dyed but not the cellulosic portion. It only stains the cellulosic portion. So the overall fastness properties of P/C blend fabrics is going to be less and also the staining of disperse dyes on cotton is more. Polyesters, and cellulose acetate (a semi-synthetic polymer) are dyed with disperse dyes. Polyamides have functional groups similar to proteins[9], so may be dyed with acid dyes or with disperse dyes. For the dyeing of acrylic fibers, basic dyes are primarily used. The general structure of polyester is shown below:
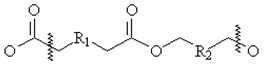 | Figure 1. Structure of Polyester; R (R1, R2….) = functional groups[9] |
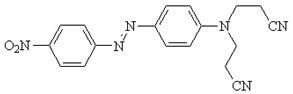 | Figure 2. Structure of disperse Dye[14] |
2. Dyeing of Cotton/Polyester Fabric
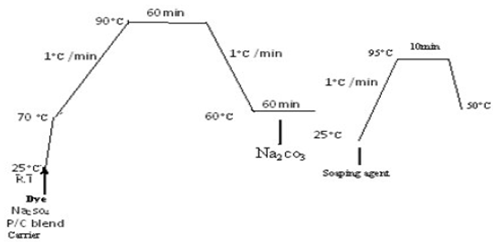
- For satisfactory dispersion in the dye batch, the dye were initially finished by mortar milling in the presence of a specially selected dispersing agent cotton/polyester fabric were dyed in Atlas dyeing machine[7, 12]at a liquor ratio of 40:1 using distilled water. The dye batch were prepared with the dye at range of dye concentrations (0.5%,1%,1.5%, 2%, and 3% O.W.F.) and with 1.5 g/l anionic Carrier (Levegal PEW Bayer Co.). The pH was then adjusted to 4, 6,7,8,9 and 10 using 10% acetic acid and 0.2 mol sodium carbonate solution. Dyeing was started at 45℃ for 15 minute, then the dye batch temperature was raised at a rate of 1.5-2℃/min to 90℃ and the dyeing continued at the desired temperature for a further 60 minute unless otherwise specified. Dyeing was commenced at 70℃ (Fig 3). The dye batch temperature was raised by 1 c/min to 90℃, maintained at this temperature for 60 minutes and cooled to 60℃.After 30 min at 60℃, 20g/l of alkali (Na2CO3) was added to effect fixation of the dye on cotton and maintained at 60℃ for further 30 min. The dyeing were rinsed and soaped at 95℃ for 15 min with 1.5 g/l soaping agent[13].
3. Testing of Characterization of P/C Blend Sample
- The amount of dye pickup of polyester/cotton fabrics during dyeing was determined spectrophotometrically using spectrophotometer (Labomed-model spectro 23 RS, USA)[16].
3.1. Test for color fastness
- The untreated and solvent-pretreated 80:20 PCFs after dyeing were tested for their wash fastness, light fastness and rub fastness using AATCC test methods (AATCC technical manual 2000). The washing fastness, was evaluated by AATCC method 61(2A) using an Atlas-Launder Ometer. Fastness to light was evaluated by AATCC method 16E using an Atlas CI 3000 + Xenon Weatherometer. The fastness to rubbing was also evaluated as per AATCC 116-1995 standards using crock meter[5].
3.2. Determination of weight loss
- The weight loss percentage of the treated fabrics was determined by measuring the weights before and after pretreatments using an electronic balance Sartorius-GD 503-Germany[13].
3.3. Abrasion Resistance
- The abrasion resistance of the fabric samples after and before solvent treatment was measured by Martindale abrasion tester as per ASTM D4966 test method.
3.4. SEM Topography
- Scanning electron microscopic studies were made on treated and untreated samples with S-3000H- Hitachi, Japan to study surface modifications if any caused by the solvent pretreatments using azeotropic mixtures. The samples were imaged with a magnification of 500X for better understanding of the inner core of the sample[9].
3.5. FTIR Analysis
- Fourier Transform infrared spectral analysis of the treated and untreated fabrics was recorded in the range of 4000–400 cm−1 using Perkin Elmer spectrometer (spectrum BX, USA) with built-in spectral matching computerized software. The fabric samples were made into individual fibers and were mounted onto the instrument for recording the spectrum.[7]
3.6. Thermal Analysis
- Thermal analysis of the untreated and solvent-mixture-pretreated 80:20 PCF was made using Perkin Elmer Pyris 6, USA at a temperature range of −50 to 400°C with a heating rate of 50℃/min under inert atmosphere of nitrogen gas at a rate of 20 mL/min[8].
3.7. X-ray Diffraction Studies
- X-Ray diffraction studies using PANalytival-mode X’Pert PRO was carried out for both untreated and solvent-treated 80:20 PCFs for determining the crystalline and amorphous region of both treated and untreated samples. The samples were analyzed by observing number of counts as a function of scattering angle[8].
3.8. Dyeing behavior of fabric
- The effect of azeotropic mixture of solvent pretreatments on the dyeing behavior of 80:20 PCF was studied by dyeing the pretreated and untreated fabrics for different dyeing time intervals (30, 45, and 60 minutes) and at different temperatures (80, 95, and 110℃).It is clear from the figures that maximum dye uptake is observed in the case of samples pretreated for 8 minutes with the solvent systems Ac-EA-Cf and Ac-MAc-nH. As the pretreatment time increases, the dye uptake is found to increase with increase in dyeing temperature and duration of dyeing. The dye uptake for the samples treated beyond 8 min was found decreasing. The change in dyeing behavior of the treated fabrics reflects changes in fiber structure of the treated fabrics caused by azeotropic mixtures of solvents. Due to solvent pretreatment, the molecular structure of the fabrics gets loosened, resulting in increased dye uptake. The improvement in the dye uptake of treated samples is probably due to the large increase in inter surface by swelling or plasticizing action, greater segmental mobility of polymer molecules, formation of micro voids, and so on. The pretreatment enabled to get better dye uptake even at a low temperature of 80°C, and in the cases where the pretreatment time is above 8 minutes, the dye uptake is found to decrease which may be due to the desorption of dye from the fabric due to irreversible swelling of the fibre. The extent of improvement in dyeing behavior was found to be different for different dyes[9].
3.9. Fastness Properties
- The results indicate that the solvent treatments involving azeotropic mixtures of solvents have slightly improved the fastness properties of the dyed polyester/cotton blended fabrics[11]. This may be due to the fact that the solvent pretreatments have improved the penetration of the dyestuff molecules into the interior of the fiber matrix and have improved the stability of dye-fiber bond[10].
3.10. Weight Loss and Abrasion Resistance Measurements
- It changes in weight and abrasion resistance of the solvent pretreated fabrics in comparison with untreated fabrics. It was found that the weight loss is very small and is dependent upon the pretreatment time. As the pretreatment time increases, there was an increase in weight loss. The abrasion resistance measurements of the treated materials show that there was a slight increase in abrasion resistance. The extent of increase in abrasion resistance was found to increase with increase in treatment duration due to increased pitting of fiber surface. However, the overall effect of solvent pretreatment has not caused any detrimental effect[9, 17].
3.11. Tearing Strength Measurements
- Tearing strength measurement of untreated and azeotropic solvent-mixture-pretreated samples showed that there is significant improvement in the tearing strength of the treated materials. In all the cases, the maximum load applied has been found to increase and the elongation percentage remains almost constant. The above changes may be due to very less influence of the solvent treatment on crystallinity index. The mechanical properties of textile fibers depend not only on the degree of crystallinity of fibers but also on the various secondary valence forces that operate in the polymer. The improvement in the strength of treated materials can be attributed to improvement in the structural order of the polymer matrix and generation of more number of crystallites, leading to improvement in the resistance power to deform the material with higher interchain bond. These observations are strengthened by the XRD and DSC results as well. The present observations are in conformity with the reports available on the effect of solvent pretreatment on polymers where, in the solvents, it does not penetrate the compact crystalline region in the polymer and therefore do not affect the strength of the polymer material[11].
3.12. FTIR Studies
- The FTIR spectrum of 80:20 PCF before and after solvent treatment was recorded to assess structural change if any made in the fiber of the alteration of existing functional groups as a consequence of azeotropic solvent mixture pretreatments. It was found, from the spectra, that the patterns are almost identical for both treated and untreated samples without any additional peaks. However, on comparing the samples treated with the two different azeotropic solvent mixtures, Ac-EA-Cf caused a slight shift in the position of the peak to a higher wave number than that treated with Ac-MAc-nH due to its higher polarity index. The extent of shift was found to be dependent on solvent pretreatment time. A broad peak at 1730 cm-1 is characteristic of carbonyl stretching of unsaturated ester. In the case of solvent treated fiber, the width of the peak had reduced and the peak value has been shifted to higher wave number, that is, 1750 cm-1. A small peak in the region between 800 and 850 cm-1 can be accounted for out-of-plane bending of aromatic ring system. The peak at 1250 cm-1 and 1300 cm-1 may be due to C-O stretching of the polymer back bone. An intense peak at 2350–2360 cm-1 can be attributed to methylene C–H stretching. The small peak close to 3000 cm-1 can be correlated to C-H stretching of aromatic ring. An interesting feature in the above-discussed spectrum was that an additional sharp small peak observed at around 3600 cm-1 corresponds to free -OH groups of cellulose component indicating that solvent treatment had increased the extent of amorphous region in the cotton component of the material. This trend was further been supported by the results of strength measurements and SEM studies. The observed small peaks between the regions 1110–1150 cm-1 were due to cellulosic component of the fiber materials[8].
3.13. Effect of pH
- Dye was introduced into the dyeing along with 200ml buffer solution at various pH values .After the dye batch temperature reached 90℃. Each 2 g cotton and polyester fiber was immersed in the liquor and kept there for 1 hr. After this the dyed sample was removed and the unreacted dye extracted with methanol. The dyed cotton and polyester were dissolved by calcium chloride/ water/ethanol mixture (1:7:2 molar ratio) and 90% formic acid respectively, cooled to room temperature and diluted to a total volume of 100ml. The concentration the solution was determined by colorimetry and the amount of dye fixed was calculated. The amount of dye removed from the batch determined by adding the amount of dye extracted to the amount of dye fixed on the fiber. The reactive /disperse dyes used gave negligible fixation on polyester fiber and so only the unfixed dye was determine by colorimetry of the residual solution[11-13].
3.14. Effect of temperature and total dye concentration
- A constant of dye was place in a dyeing vessel along with 200 ml buffer solution (0.01ml acetic acid 35%/sodium acetate of solution at pH 6)[12]. After the dye batch temperature achieved a constant value (75,85 or 90℃), both the cotton/polyester fabric were immersed in the dye batch where they remained for 0-4.5 hr. The cotton and polyester fiber were also dyed in a dye batch (pH 6) containing various amounts of reactive/disperse dye for 1 hr at 90℃ (exhaustion and fixation of dye on the cotton and polyester fibers were determined as described previously). Cotton and polyester fabrics were dyed by disperse/reactive dyes (dyes1 and dye 2 respectively) in the dye batch at various pH values. Both dyes were exhaustion and fixed over a pH range 6to 8, but only a very small proportion of dye 1 was exhaustion onto the polyester. Dye 1 was taken, up more by cotton and polyester, and even less of dye 2. The more hydrophobic dye 1 was taken, up more by cotton and polyester than dye 2[16].
3.15. XRD Studies of 80:20 polyester-cotton fabric (PCF)
- X-ray diffraction studies on a polymer are mainly concerned, with study of crystalline, amorphous, and semicrystalline regions/phases, which are responsible for observing their respective electrical and mechanical properties. X-ray diffraction pattern of most polymers contains sharp as well as broad and diffuse peak. The sharp peak corresponds to crystalline regions; the diffuse and the broad ones refer to amorphous region. The interaction of solvent with polymer results in recrystallization and decrystallization of the corresponding polymer contents. The results from XRD reveals that the solvent treatment disturbs the amorphous region of the fabric material used in the present study, probably creates more cavity and pores resulting in the opening up of the structured assembly enhancing more dye uptake when compared with the untreated. Increase in pretreatment duration causes much pronounced effect on the treated materials, which leads to improved dye uptake. The above observation is supported by the weight loss and tearing strength measurements wherein no much loss in weight and strength was observed. The effect of azeotropic mixture of solvent pretreatments on the dyeing behavior of 80:20 PCF was studied[11, 14]. As the pretreatment time increased, the dye uptake was found to increase. The slight improvement in the fastness properties of the pretreated fabrics revealed that the treatment has not affected the dye-fiber bond and the improvement in fastness is due to improved dye pickup and dye-fiber bond formation. The abrasion resistance measurements of the treated materials show that there was a small increase in abrasion resistance of solvent pretreated samples up to 6 minutes pretreatment time. Prolonged solvent pretreatments led to decrease in abrasion resistance when treated for more than 6 minutes. As the time of pretreatment increased, the weight loss of the fabric was also found to increase. SEM studies showed that the azeotropic solvent mixtures attacked the entire surface of the fabric materials and caused erosion. As the time of solvent treatment increased, erosion propagated into the fiber structure resulting in the formation of elongated pits or cavities on the surface. FTIR analysis of treated and untreated fabrics showed that there was no structural change or introduction of any functional groups or alteration of the existing groups in the case of solvent-treated materials used in the study. It is also concluded from the XRD and DSC analysis that the solvent treatment has disturbed the crystalline distribution probably by creating more cavity and pores resulting in opening up of the structured assembly. The improvement in the dye uptake of solvent-treated fabrics is due to large increase in intersurface area by swelling and greater segmental mobility of polymer molecules[13].
4. Thermodynamic View: an Approach to Reach the Dyeing Equilibrium
- DSC curves of 80:20 PCF treated with azeotropic solvents mixtures and untreated samples shows the starting temperature and peak melting temperature are noted. The final melting temperature corresponds to the melting of the most stable crystallite whereas the peak melting temperature is taken as the temperature at the maximum of melting endothermic. The starting temperature is the starting of the melting endotherm and can be regarded as the melting of the smallest crystallite in the sample. The DSC thermo grams of solvent pretreated samples obtained are found to be almost identical with that of untreated samples with small changes in terms of starting temperature, peak temperature, and melting temperature[13]. However, maximum heat flow has increased considerably for solvent pretreated samples due to solvent-induced crystallization. During the interaction of the polymer with the solvent, the solvent enters into the amorphous region of polymer structure, weakens polymer-polymer interaction, replaces it with polymer-solvent interaction, induces extensive segmental motion, and lowers the effective glass transition temperature of material. The polymer chains rearrange themselves into a lower free energy state. This induces crystallization even in the swollen state. The interaction of solvent with the polymer may be of two types, namely, intercrystalline interaction and intracrystalline interaction[12]. In the case of intercrystalline interaction, the solvent penetrates inside the amorphous region only. The polymer chains within this region are under lower stress, and this generally results in the rearrangement of molecular chains. In this case, crystallization takes place in the swollen state and crystalline areas of the sample increase. On the other hand, in the case of intracrystalline interaction, the interacting solvent penetrates inside the crystalline region, decrystallizes the sample, and affects higher lateral order parts of the fiber. In the present study, the interaction of solvent with the fiber material is found to be intercrystalline interaction. This is evident from the considerable increase in the melting heat for solvent-treated samples due to solvent-induced crystallization[12, 15]. It is further supported by the observed small increase in starting temperature, peak temperature, and melting temperature of the treated samples.
5. Conclusions
- The dyeing of PCF is a fascinating and tremendous job in the textile processing, but with the help of proper guidance of dye selection, dye combination analysis[8], pre-diagnosis of dyeing machinery[1], dyeing auxiliaries the dyeing parameters can be optimized. The tensile strength[11], surface smoothness of polyester/cotton blend fabric can be significantly improved by the right establishment of kinetic dyeing thermodynamics and which leads to the excellency of a new dyeing era with almost zero defect in shed[13].
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML