-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Textile Science
p-ISSN: 2325-0119 e-ISSN: 2325-0100
2012; 1(6): 101-105
doi: 10.5923/j.textile.20120106.07
Effect of Mercerization and Benzoyl Peroxide Treatment on Morphology, Thermal Stability and Crystallinity of Sisal Fibers
Vijay K. Kaushik1, Anil Kumar1, Susheel Kalia2
1Department of Chemistry, Haryana Institute of Technology, Asodha, Bahadurgarh, 124507, Haryana, India
2Department of Chemistry, Shoolini University of Biotechnology and Management Sciences, Bajhol , 173 229, Dist. Solan (H.P.) India
Correspondence to: Vijay K. Kaushik, Department of Chemistry, Haryana Institute of Technology, Asodha, Bahadurgarh, 124507, Haryana, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Studies on the use of natural fibers as replacement to man-made fiber in fiber-reinforced composites have increased and opened up further industrial possibilities. Natural fibers have the advantages of low density, low cost, and biodegradability. However, the main disadvantages of natural fibers in composites are the poor compatibility between fiber and matrix and the relative high moisture sorption. Therefore, chemical treatments are considered in modifying the fiber surface properties. In this study, Sisal fibers were modified using alkali and benzoyl peroxide solution of different concentration for different time intervals. Morphological changes, thermal stability and crystallinity of fibers were investigated using scanning electron microscope (SEM), TGA and XRD technique. Thermal stability of sisal fibers were decreased on mercerization. Whereas, sisal fibers treated with benzoyl peroxide the enhanced thermal stability. In case of XRD studies, sisal fibers show enhanced crystallinity.
Keywords: Sisal fiber, Benzoyl Peroxide, SEM, XRD, Crystallinity
Cite this paper: Vijay K. Kaushik, Anil Kumar, Susheel Kalia, "Effect of Mercerization and Benzoyl Peroxide Treatment on Morphology, Thermal Stability and Crystallinity of Sisal Fibers", International Journal of Textile Science, Vol. 1 No. 6, 2012, pp. 101-105. doi: 10.5923/j.textile.20120106.07.
Article Outline
1. Introduction
- Effect of chemical treatments on surface morphology, thermal behavior and structure of natural fibers was reported by various authors[1-4]. Chemical treatments remove the lignin from surface of natural fibers and fiber surface becomes rough. Chemical treatments also reduce the number of free hydroxyl groups of the cellulose, which results in the reduction of the polarity of the cellulose molecules and enhance the compatibility with hydrophobic polymer matrices[5]. Alkaline treated sisal fibers were dipped in permanganate solution at concentrations of 0.033, 0.0625, and 0.125% in acetone for 1 minute[6]. As a result of permanganate treatment, the hydrophilic tendency of the fibers was reduced. Peroxide treatments include different concentration of benzoyl peroxide or dicumyl peroxide in acetone solution for about different minutes after alkali pretreatment[7-9]. Pre-treatments of the fiber can clean the fiber surface, chemically modify the surface, stop the moisture absorption process and increase the surface roughness[10]. As the natural fibers bear hydroxyl groups from cellulose and lignin, therefore, they are amenable to modification. The hydroxyl groups may be involved in thehydrogen bonding within the cellulose molecules there by reducing the activity towards the matrix. Chemical modifications may activate these groups or can introduce new moieties that can effectively interlock with the matrix. Mercerization, isocyanate treatment, acrylation, permanganate treatment, acetylation, silane treatment and peroxide treatment with various coupling agents and other pre-treatments of natural fibers have achieved various levels of success in improving fiber strength[3]. The interest in using natural flax fibers as reinforcement in biocomposites has increased dramatically and also represents one of the most important uses. Cellulosic fibers are hygroscopic in nature; moisture absorption can result in swelling of the fibers which may lead to micro-cracking of the composite and degradation of mechanical properties. This problem can be overcome by treating these fibers with suitable chemicals to decrease the hydroxyl groups which may be involved in the hydrogen bonding within the cellulose molecules. A number of fiber surface treatments like silane treatment, benzoylation and peroxide treatment were carried out which may result in improved mechanical performance of the fiber and composite[2,3]. The possibility of forming mechanical and chemical bonding at the fiber surface is mainly dependent on the surface morphology and chemical composition of the fibers. Therefore, the microscopic analysis of fiber surface topology and morphology is of utmost importance in fibrous compositesIn the present paper, we have reported the mercerization and peroxide treatments of sisal fibers using sodium hydroxide and benzoyl peroxide of different concentration and for different time interval. Effect of these treatments on morphology, crystallinity and thermal stability of sisal fibers is meagerly reported in literature.
2. Experimental
2.1. Materials
- Sisal fibers were obtained from sisal research station, Bamra - Sarnbalpur, Orissa. Benzoyl peroxide, NaOH and acetone of 99.5% purity were supplied by S.D. Fine, India. Before chemical treatment sisal fiber were previously cut in definite size, washed with distill water and dried at 60℃ followed by soxhlet extraction of sisal fibers with acetone for 72 hours and purified fibers were dried at room temperature.
2.2. Scanning Electron Microscopy (SEM)
- Scanning electron microscopic studies of sisal fibers and all its chemically treated fibers were carried out on Electron Microscopy Machine (LEO 435 VP, UK). Since cellulose has a non conducting behavior so it was gold plated to have an impact.
2.3. Thermo Gravimetric Analysis (TGA)
- Thermal analysis was carried out in nitrogen atmosphere at a heating rate of 10℃ /min by (Pyris Diamond) thermal analyzer (Perkin Elmer, USA) Nitrogen is supplied at a rate of 200 ml per minute.
2.4. X-Ray Diffraction (XRD) Studies
- X-ray diffraction studies were performed under ambient condition on X-ray diffractometer (D8 Advance, Brucker, AXS, Germany) using Cu Kα (1.5418Ǻ) radiation, Ni filter and scintillation counter as 40 kV and 30 mA on rotation between 5˚ to 50˚ at 2θ scale at 1 second step size. Percentage crystallinity and crystallinity index was calculated as followed
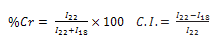 Where I22 and I18 are the crystalline and amorphous intensities at 2θ scale close to 22˚ and 18˚, respectively.
Where I22 and I18 are the crystalline and amorphous intensities at 2θ scale close to 22˚ and 18˚, respectively.2.5. Alkali Treatment
- Sisal fiber were soaked in 5%, 10% and 15% (By Weight) solution of sodium hydroxide for 4 hr. at room temperature. After treatment, fibers were thoroughly washed with distilled water and dried in hot air oven at 80℃ for 24 hours. Reaction is shown in figure 1.
2.6. Peroxide Treatment
- The peroxide treatment was carried out on an alkali pretreated sisal fiber using benzoyl peroxide at a fixed concentration for different time intervals, reaction shown in figure 2. Sisal fibers were treated with 5% benzoyl peroxide in acetone for 30 and 45 minutes. After treatments, fibers were thoroughly washed with distilled water and dried in hot air oven at 80℃ for 24 hours
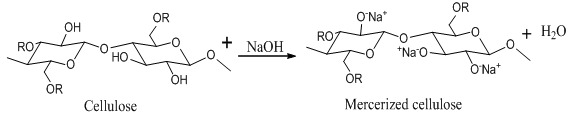 | Figure 1. Reaction of cellulose fiber with NaOH |
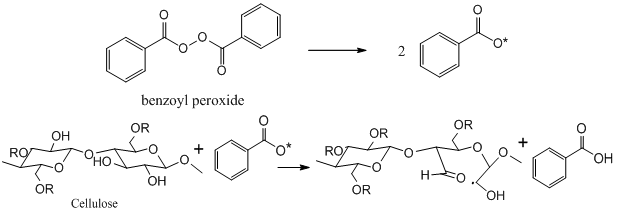 | Figure 2. Showing reaction of Sisal fiber with benzoyl peroxide |
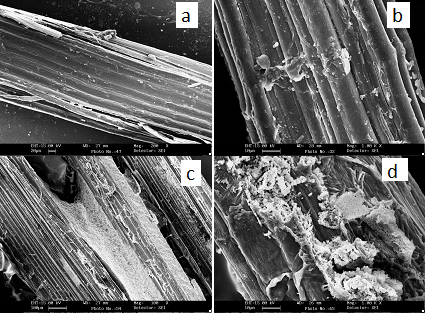 | Figure 3. SEM of (a) Raw fiber, (b) Alkali Treated 5% for 4 hrs. (c) Alkali Treated 10% for 4 hrs. (d) Alkali Treated 15% for 4 hrs |
 | Figure 4. SEM of Benzoyl peroxide Treated (e) 30 minute (f) 45 minute |
3. Results and Discussion
3.1. SEM Analysis
- Scanning electron microscopic (SEM) provide an excellent technique for the study of surface morphology of raw and chemically modified sisal fibers. It has been observed that surface of raw sisal fibers differs in smoothness and roughness than chemically treated sisal fibers. These micrographs clearly showed the difference in their surface morphology. The raw fiber (Fig. 3a) surface is very smooth in comparison to treated fibers. Figure 3b-3d showing mercerized fibers while figure 4e-4f showing BP treated fibers.
3.2. Thermal Analysis (TGA)
- TGA of raw fiber and chemical treated sisal fibers were carried out at a rate of 10℃/ min in nitrogen as a function of percentage weight loss versus temperature. The initial and final decomposition temperatures of raw fiber were 249℃ and 370℃ respectively with percentage weight loss of 9.7% and 70.5% respectively. The IDT for alkali, and benzoyl peroxide treated fibers are 220, 230, 225, 250 and 250℃ respectively and final decomposition temperatures are 329, 324, 317, 372 and 350℃ respectively as shown Table 1.First stage decomposition is due to primary changes i.e. breakdown of hemicellulose, glycosidic linkage and loss of moisture whereas the second stage of decomposition is due to cellulosic and lignin degradation. It is shown in Figure 5 and 6.
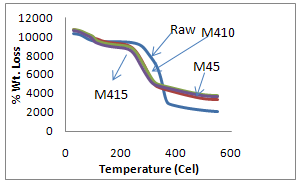 | Figure 5. TGA Plot of Raw and Mercerized sisal fibers |
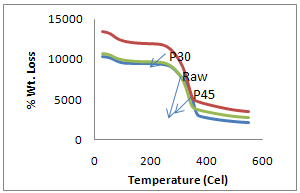 | Figure 6. TGA Plot of Raw and Peroxide treated sisal fibers |
3.3. XRD Analysis
- X-ray diffraction studies were performed on X-ray diffractometer. It is evident from Table 2 that raw fiber at 2θ scale gave peaks at 22.0 and 18.0 with relative intensity is 877 and 346 respectively. Percentage crystallinity (%Cr) and crystallinity index (C.I.) of raw sisal fiber are 71.7 and 0.60 respectively whereas percentage crystallinity of alkali treated fibers (5%, 10% and 15% for 4 Hrs) were 74.3, 62.0 and 51.2 Whereas crystallinity index of alkali treated fibers are 0.65, 0.40, and 0.40 respectively and percentage crystallinity of peroxide treated fiber (30 and 45 minutes) were 71.0 and 72.0 and crystallinity index were 0.60 and 0.61 respectively. The counter reading at peak intensity at 22˚ is said to represent the crystalline material and the peak intensity at 18˚ corresponds to the amorphous material in cellulose [11-12]. Percentage Crystallinity[13] and crystalline index[14-15] were calculated as follow:
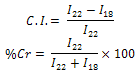
|
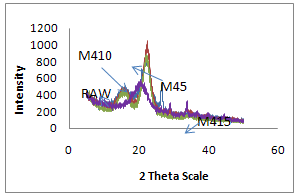 | Figure 7. XRD Plot of Raw and Mercerized sisal fibers |
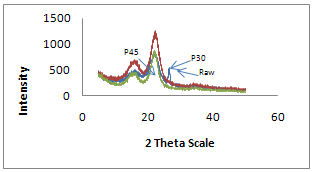 | Figure 8. XRD Plot of Raw and Peroxide treated sisal fibers |
4. Conclusions
- It is believed that the increase in the crystallinity index obtained by X-ray diffraction is in actual fact an increase of the order of the crystallite packing rather than in increase in the intrinsic crystallinity. Morphology of sisal fiber was changed by chemical treatments. The surface of sisal fiber becomes rougher after treatments in comparison with smooth and clear surface of raw sisal fibers. The removal of surface impurities on plant fibers may be an advantage for fiber to matrix adhesion as it may facilitate both mechanical interlocking and the bonding reaction due to the exposure of the hydroxyl groups to chemicals such as resins and dyes.
ACKNOWLEDGEMENTS
- Authors would like to thanks Institute instrumentation centre, Indian institute technology Roorkee and Singhania university, Rajasthan for providing all the instrumentation facilities and provide results well in time. We would also like to thanks all the faculty members of chemistry research lab, Haryana institute of technology, Haryana for helping us in carried out the whole research works.
References
| [1] | Kalia, S., Kaith, B.S., Kaur, I., “Pre treatments of natural fibers and their application as reinforcing material in polymer composites—a review”, Wiley, Polym. Engg. Sci., 49, 1253-1272, 2009. |
| [2] | Kalia S., Kaushik V.K., Sharma R.K., “Effect of Benzoylation and Graft Copolymerization on Morphology, Thermal Stability, and Crystallinity of Sisal Fibers” Taylor & Francis, J. Nat. Fib. 8, 1, 27- 38, 2011. |
| [3] | Li. X., Tabil, L.G., Panigrahi, S., “Chemical treatments of natural fiber for use in natural fiber-reinforced composites: a review”, Springer, J. polym. Environ., 15, 25-33, 2007. |
| [4] | Kalia S., Vashistha S., “Surface Modification of Sisal Fibers (Agave sisalana) Using Bacterial Cellulase and Methyl Methacrylate”, Springer, J. Polym. Environ., 20,142–151, 2012. |
| [5] | Calado, V., Barreto, D.W., D’almeida, J.R.M., “The effect of a chemical treatment on the structure and morphology of coir fibers”, Springer, J. mat. Sci. lett., 19, 2151-2153, 2000. |
| [6] | Sreekala, M.S., Kumaran, M.G., Joseph, S., Jacob, M., Thomas, S., “Oil palm fibre reinforced phenol formaldehyde composites: influence of fibre surface modifications on the mechanical performance”, Springer, Appl. Compos. Mater., 7, 295-329, 2000. |
| [7] | Paul, A., Joseph, K., Thomas, S., “Effect of surface treatments on the electrical properties of low-density polyethylene composites reinforced with short sisal fibers”, Science direct, Compos. Sci. Technol., 57, 67-79, 1997. |
| [8] | Sreekala, M.S., Kumaran, M.G., Thomas, S., “Water sorption in oil palm fiber reinforced phenol formaldehyde composites”, Elsevier, Compos. Part A: Appl. Sci. Manuf., 33, 763-777, 2002. |
| [9] | Wang, B., Panigrahi, S., Tabil, L., Crerar, W., “Pre-treatment of flax fibers for use in rotationally molded biocomposites”, Sage publication, J. Reinf. Plast. Compos., 26, 447-463, 2007. |
| [10] | Kaith, B.S., Kalia, S., “Synthesis and characterization of graft co-polymers of flax fiber with binary vinyl monomers”, Taylor and Francis, Int. J. Polym. Analys. Charact., 12, 401-412, 2007. |
| [11] | Singh, V., Tiwari, A., Tripathi, D. N., Sanghi, R., “Grafting of polyacrylonitrile onto guar gum under microwave irradiation”, Wiley, J. Appl.Polym. Sci., 92, 1569 – 1575, 2004. |
| [12] | Singh, V., Tiwari, A., Tripathi, D. N., Sanghi, R.,” Microwave promoted synthesis of chitosan-graft-poly (acrylonitrile)”, Wiley, J. Appl. Polym. Sci., 95, 820–825, 2004. |
| [13] | Kaith, B. S., Singha, A. S., Gupta, S. K., “Graft copolymerization of Flax fibres with binary vinyl monomer mixtures and evaluation of swelling, moisture absorbance and thermal behaviour of the grafted fibres”, A. A. Balkema Publishers J. Polym. Mater., 20, 195 –199, 2003. |
| [14] | Princi, E., Vicini, S., Pedemonte, E., Mulas, A., Franceschi, E., Luciano, G., Trefiletti, V., “Thermal analysis and characterisation of cellulose grafted with acrylic monomers”, Elsevier, Thermochim. Acta, 425, 173–179, 2005. |
| [15] | Kaith, B. S., Singha, A. S., Kalia Susheel, “Mechanical Properties of Raw Flax and Flax-g-poly (MMA) Reinforced Phenol-Formaldehyde Composites”, Springer, Int. J. Plast. Tech., 10, 572-587, 2006. |
| [16] | Aboul-Fadl, S.M., Zeronian, S.H., Kamal, M.M., Kim, M.S., Ellison, M.S., “ Effect of mercerisation on the relation between single fibre mechanical properties and fine structure for different cotton species”, SAP, USA, Textile Research Journal, vol. 55, 461 – 469,1985. |
| [17] | Geethamma, V.G., Joseph, R., Thomas, S., “Short Coir fibre Reinforced Natural Rubber Composites: Effects of Fibre |
| [18] | Length, Orientation and Alkali Treatmant”, Wiley, J. App. Poly. Sci., vol. 55, 583 – 594, 1995. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML