-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Textile Science
p-ISSN: 2325-0119 e-ISSN: 2325-0100
2012; 1(6): 62-68
doi: 10.5923/j.textile.20120106.02
Synthesis of Some New 5-Arylazothiazole Derivatives as Disperse Dyes for Dyeing Polyester Fibers
Mohamed E. Khalifa1, 2, Ehab Abdel-Latif3, Fathy A. Amer3, Mohamed A. Metwally3
1Department of Chemistry, Faculty of Science, Taif University, Taif 21974, Kingdom of Saudi Arabia
2Higher Institute for Engineering and Technology, New Damietta, Egypt
3Department of Chemistry, Faculty of Science, University of Mansoura, Mansoura, Egypt
Correspondence to: Mohamed E. Khalifa, Department of Chemistry, Faculty of Science, Taif University, Taif 21974, Kingdom of Saudi Arabia.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
A series of 5-arylazo-2-(arylidenehydrazino)-2-thiazolin-4-one dyes 3 and 4 was prepared by cyclocondensation of hydrazonoyl bromides 2 with various thiosemicarbazone derivatives 1. While the synthesis of 3-amino-N-(5-arylazo-2-thiazolyl)-thieno[2,3-b]pyridine-2-carboxamide dyes 8 was achieved by the reaction of 2-(N-chloroacetyl)-5-arylazo-thiazole derivatives 5 with 4,6-dimethyl-2-mercaptonicotinonitrile followed by heterocyclization of the obtained sulfide derivatives 7 in a solution of ethanol containing sodium ethoxide. These dyes were applied as disperse dyes for dyeing polyester fibers and their characteristics and fastness properties have been measured. The dyed fibers exhibit very good to excellent washing, perspiration and sublimation fastness properties, with little variation in good to excellent rubbing and light fastness.
Keywords: Thiosemicarbazone, Thiazolidin-4-one, Mercaptonicotinonitrile, Azo Coupling, Disperse Dyes, Polyester Fibers, Fastness Properties
Cite this paper: Mohamed E. Khalifa, Ehab Abdel-Latif, Fathy A. Amer, Mohamed A. Metwally, "Synthesis of Some New 5-Arylazothiazole Derivatives as Disperse Dyes for Dyeing Polyester Fibers", International Journal of Textile Science, Vol. 1 No. 6, 2012, pp. 62-68. doi: 10.5923/j.textile.20120106.02.
Article Outline
1. Introduction
- The chemistry of 2-thiazolines, including new methodologies for their preparation, and recent applications, such as their growing use in organic synthesis in the biological field and asymmetric catalysis as ligands has been recently reviewed[1]. 2-Aminothiazoles are known mainly as biologically active compounds with a broad range of activity and as intermediates in the synthesis of antibiotics and dyes[2]. Several papers have been published on the use of these compounds as antimicrobial[3], antifungal[4], anti-inflammatory activity[5], anesthetic[6] and antiviral drugs[7]. 2-Aminothiazoles and their derivatives are also used in the syntheses of various types of dyes[8-11]. In continuation of our previous studies[9, 12-15] on the synthesis of a variety of thiazole derivatives from the readily obtainable cheapest starting materials for dyeing polyester fabrics, we focused on the synthesis of several new S/N heterocyclic azo dyes and their applications as azo-disperse dyes for dyeing polyester fibers. These sulfur and/or nitrogen heterocyclic azo dyes provide strong shades that range from yellow, orange, red, and brown colors.
2. Results and Discussion
- Heating of thiosemicarbazones 1 with ethyl arylhydrazonobromoacetates 2 in ethanol containing anhydrous sodium acetate afforded the formation of the corresponding 2-(benzylidenehydrazino)-5-arylazo-thiazolidin-4-one derivatives 3 and 4 (Scheme 1). The chemical structure of dyes 3 and 4 was based on their elemental analyses and spectral data. The IR spectra of the synthesized dyes 3 and 4 displayed absorption bands corresponding to NH in the region 3260-3189 cm-1 in addition to the characteristic absorption frequencies in the region 1712-1697 cm-1 corresponding to the carbonyl group. The 1H NMR spectrum of 3b revealed a singlet signal at δ 2.4 for the methyl protons (Ar-Me), multiplet in the region δ 7.1-7.9 for the aromatic and NH protons, singlet at δ 8.5 for the methine proton (CH=N), in addition to singlet for the hydrazono proton (C=N-NH) at δ 11.5 ppm. The 2-(N-chloroacetyl)-5-arylazo-thiazole derivatives 5[15] were reacted with 4,6-dimethyl-2- mercaptonicotinonitrile 6 by refluxing in acetone containing sodium carbonate to yield the corresponding sulfide derivatives 7, which underwent cyclization on heating in a solution of ethanol containing sodium ethoxide to afford the corresponding thieno-[2,3-b]pyridine derivatives 8 (Scheme 2). The chemical structures of 7 and 8 were established on the basis of their elemental analyses and spectral data. The IR spectrum of 7a is characterized by the presence of strong absorption bands at 3333 cm-1 for the NH stretching, 2221 cm-1 corresponding to the nitrile group and 1699 cm-1 corresponding to the carbonyl group. The IR spectrum of 8a clearly indicated the lack of cyano absorption band and revealed the characteristics of NH2 absorption bands at 3177, 3280 cm-1 in addition to the carbonyl absorption band at 1632 cm-1. The strong decrease in the carbonyl absorption frequencies is attributed to the highly chelated intramolecular H-bond structure. The 1H NMR spectrum of 7b is characterized by the presence of three singlet signals at δ 2.4, 2.5 and 2.6 corresponding to three methyl protons, singlet at δ 4.2 for the methylene protons, singlet at δ 7.0 for pyridine C5-H proton and multiplet at δ 7.2-7.9 for aromatic protons. The 1H NMR spectrum of 8b confirmed the lack of the singlet signal that characterized the methylene protons and showed three singlet signals corresponding to the three methyl protons at δ 2.4, 2.8 and 3.0, in addition to the singlet signal at δ 7.3 for the pyridine C5-H proton and multiplet signal at δ 7.4-7.9 for the aromatic protons.
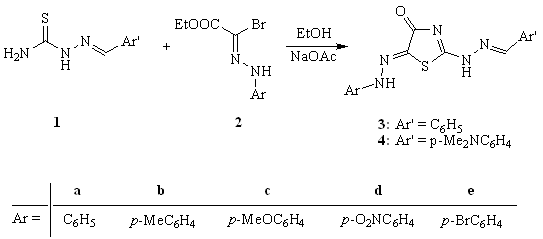 | Scheme 1. Synthesis of 2-(benzylidenehydrazino)-5-arylazo-thiazolidin-4-one derivatives 3 and 4 |
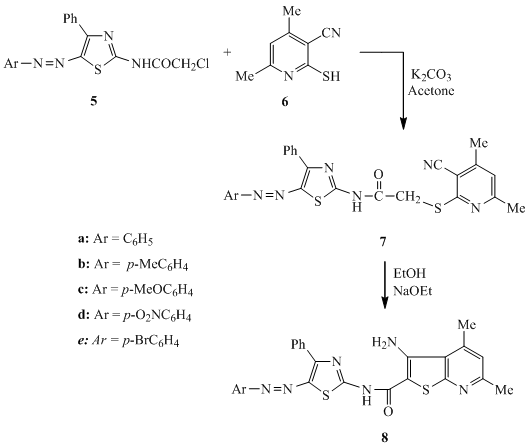 | Scheme 2. Synthesis of thieno-[2,3-b]pyridine derivatives 8 |
2.1. Absorption Spectral Characteristics
| ||||||||||||||||||||||||||||||
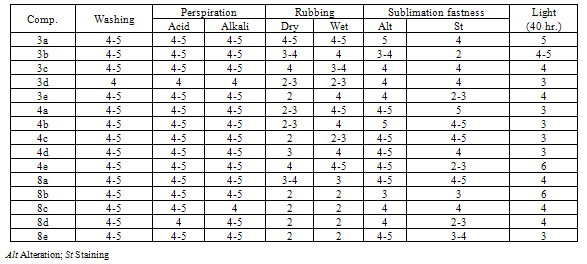
2.2. Dyeing and Fastness Properties
- The synthesized disperse dyes under investigation were applied to polyester fibers at 2% shade by high-temperature pressure technique (130℃) where a range of color shades has been obtained as the visual color shades varied from yellow, golden yellow, orange, reddish brown to brown. Generally, variation in color of these dyes results from the alternation in the diazonium and coupling components. The dyes on polyester fibers were evaluated in terms of their fastness properties using standard method[16] and given in table (2), where fastness to light, sublimation and perspiration was assessed in accordance with AATCC-15 (1985), rubbing fastness test was carried out with a crockmeter (Atlas) in accordance with AATCC-88 (1988) and wash fastness test in accordance with IS: 765-1979.
|
2.3. Color Assessment
- The color on polyester fibers is expressed in terms of CIELAB values (table 3) and the following CIELAB coordinates are measured, lightness (L*), chroma (C*), hue angle from 0º to 360º (h), (a*) value represents the degree of redness (positive) and greenness (negative) and (b*) represents the degree of yellowness (positive) and blueness (negative). A reflectance spectrophotometer (Gretag Macbeth CE 7000a) was used for the colorimetric measurements on the dyed samples. K/S values given by the reflectance spectrometer are calculated at λmax and are directly correlated with the dye concentration on the dye substrate according to the Kubelka–Munk equation: K/S = (1-R)2/2R, where K = absorbance coefficient, S = scattering coefficient, R = reflectance ratio. The application of the dyes 3, 4 and 8 on polyester fibers has shown that such dyes have good affinity to polyester fibers. As shown in table 3, the color hue of the applied dyes 3 and 4 on polyester fibers is shifted to the greenish direction on the red-green axis as indicated from the negative value of a*, whereas the dyes 3, 4 and 8 represent degrees of redness to yellowness color hue as indicated from the positive values of a* and b*. These results are in line with the previously reported by Műller[17] on the effect of substituent in the dye structure and hue.
3. Conclusions
- A set of 15 disperse dyes 3, 4 and 8 were synthesized by azo coupling. All of them were investigated for their dyeing characteristics on polyester fibers. The electronic absorption spectra give variable hues from yellow to brown on polyester fibers. The dyed fibers exhibit very good to excellent washing, perspiration and sublimation fastness properties with little variation in the good to excellent rubbing fastness. The degree of levelness after washing is indicative of good penetration and the excellent affinity of these dyes for polyester fiber. This in combination with the ease of preparation makes them particularly valuable.
4. Experimental
4.1. Materials and Instrumentation
- Microanalysis of the elements: carbon and hydrogen were determined at Microanalytical Laboratories, Faculty of Science, Mansoura, Cairo and Alexandria Universities. All melting points are in degree centigrade and are uncorrected. Infrared spectra were recorded on a Perkin Elmer 14 spectrometer using potassium bromide Waffer technique. 1H NMR spectra were measured on a Bruker WP 300 in CDCl3, DMSO or CF3COOD as solvent, using TMS as an internal standard. Mass spectra were recorded on a Finnigan MAT 212 instrument. The substrate used for dyeing (100% polyester fiber) was kindly provided by Misr Beida Dyers company, Alexandria, Egypt, a product of Misr For Synthetic Fibers Company, Kafr El-Dawar, Egypt. The colorimetric measurements for the dyed polyester fibers were carried out using a reflectance spectrophotometer (GretagMacbeth CE 7000a). Fastness to washing was carried out using the automatic launder Rotadyer (sponsored by the British Standard Institute – Society of Dyers and Colourists, fastness to perspiration was assessed according to the test sponsored by the (BSS), fastness to rubbing was carried out according to the standard method of testing (BSS) using Crockmeter of Electric Hungarian FD-17 type, fastness to sublimation was carried out using the Electric Japanese Thermotester T-10 type and fastness to light was carried out using the “ Weather-o-meter” (Atlas Electric Devices Co. USA), AATCC standard test method.
4.2. Dyestuff Synthesis
- Synthesis of 2 - (Benzylidenehydrazino)-5-arylazothiazolidin -4-one dyes 3 and 4A mixture of equimolar amounts of thiosemicarbazones 1 (0.005 mol) and ethyl arylhydrazono-bromoacetates 2 (0.005 mol) in ethanol containing anhydrous sodium acetate (0.5 gm) was refluxed for 2 h. The products separated on cooling was collected by filtration and washed several times with cold water, dried and recrystallized from ethanol3a, Yield 62%; yellow; mp 162-164℃; IR (KBr) υ= 3210 (NH), 1712 (CO) and 1611 (C=N) cm-1. Anal. calcd. for C16H13N5OS (323.37): C, 59.43; H, 4.05; N, 21.66. Found: C, 59.51; H, 4.18; N, 21.77. 3b, Yield 30%; orange; mp 118-119℃; IR (KBr) υ= 3227 (NH), 1710 (CO) and 1638 (C=N) cm-1. 1H NMR (DMSO): δ/ppm = 2.4 (s, 3H, CH3), 7.1-7.9 (m, 10H, Ar-H and NH), 8.5 (s, 1H, CH=N), 11.5 (s, 1H, C=N-NH). Anal. calcd. for C17H15N5OS (337.4): C, 60.52; H, 4.48; N, 20.76. Found: C, 60.71; H, 4.55; N, 20.87. 3c, Yield 77%; brown; mp 180-183°C; IR (KBr) υ= 3238 (NH), 1699 (CO) and 1644 (C=N) cm-1. 1H NMR (DMSO): δ/ppm = 3.8 (s, 3H, OCH3), 6.9-7.8 (m, 10H, Ar-H and NH), 8.3 (s, 1H, CH=N), 11.2 (s, 1H, C=N-NH). Anal. calcd. for C17H15N5O2S (353.4): C, 57.78; H, 4.28; N, 19.82. Found: C, 57.71; H, 4.32; N, 19.76 3d, Yield 68%; yellow; mp >265℃; IR (KBr) υ= 3260 (NH), 1708 (CO) and 1647 (C=N) cm-1. MS (M+; EI): m/z (%) = 368 (100). Anal. calcd. for C16H12N6O3S (368.37): C, 52.17; H, 3.28; N, 22.81. Found: C, 52.24; H, 3.18; N, 22.84.3e, Yield 20%; yellow; mp 249- 251℃; IR (KBr) υ= 3189 (NH), 1707 (CO) and 1626 (C=N) cm-1. Anal. calcd. for C16H12BrN5OS (402.27): C, 47.77; H, 3.01; N, 17.41. Found: C, 47.71; H, 3.08; N, 17.34. 4a, Yield 58%; yellow; mp 214- 215℃; IR (KBr) υ= 3216 (NH), 1702 (CO) and 1623 (C=N) cm-1. Anal. calcd. for C18H18N6OS (366.44): C, 59.00; H, 4.95; N, 22.93. Found: C, 59.11; H, 4.88; N, 23.10. 4b, Yield 38%; orange; mp 198- 201℃; IR (KBr) υ= 3232 (NH), 1697 (CO) and 1634 (C=N) cm-1. 1H NMR (DMSO): δ/ppm = 2.4 (s, 3H, CH3), 3.0 (s, 6H, 2CH3), 6.8-7.8 (m, 9H, Ar-H and NH), 8.3 (s, 1H, CH=N), 11.1 (s, 1H, C=N-NH). Anal. calcd. for C19H20N6OS (380.47): C, 59.98; H, 5.30; N, 22.09. Found: C, 60.09; H, 5.42; N, 22.17. 4c, Yield 22%; orange; mp 197- 198℃; IR (KBr) υ= 3224 (NH), 1694 (CO) and 1642 (C=N) cm-1. 1H NMR (DMSO): δ/ppm = 3.0 (s, 6H, 2CH3), 3.8 (s, 3H, OCH3), 6.9-7.8 (m, 9H, Ar-H and NH), 8.4 (s, 1H, CH=N), 11.2 (s, 1H, C=N-NH); Anal. calcd. for C19H20N6O2S (396.47): C, 57.56; H, 5.08; N, 21.20. Found: C, 57.71; H, 5.20; N, 21.36. 4d, Yield 18%; brown; mp 174- 176℃; IR (KBr) υ= 3197 (NH), 1709 (CO) and 1644 (C=N) cm-1. MS (M+; EI): m/z (%) = 411 (82). Anal. calcd. for C18H17N7O3S (411.44): C, 52.55; H, 4.16; N, 23.83. Found: C, 52.34; H, 4.28; N, 23.94.4e, Yield 45%; orange; mp 136- 138°C; IR (KBr) υ= 3191 (NH), 1710 (CO) and 1640 (C=N) cm-1. Anal. calcd. for C18H17BrN6OS (445.34): C, 48.55; H, 3.85;N, 18.87. Found: C, 47.71; H, 3.78; N, 18.81. Synthesis of 2-(3-cyano-4,6-dimethylpyridin-2-ylthio)-N- (5-arylazo-4-phenylthiazol-2-yl)acetamides 7A mixture of 2-(N-chloroacetyl)-5-arylazo-thiazole derivatives 5 (0.01 mol), 4, 6- dimethyl-2-mercaptonicotinonitrile 6 (0.01 mol), and anhydrous potassium carbonate (0.01 mol) in acetone (30 ml) was refluxed for 4 hours. The excess acetone was evaporated under reduced pressure. The residue was washed with water and recrystallized from ethanol.7a, Yield 25%; yellow; mp 265- 268℃; IR (KBr) υ= 3333 (NH), 2221 (CN) and 1697 (CO) cm-1. 1H NMR (CDCl3/CF3COOD): δ/ppm = 2.4 (s, 3H, CH3), 2.50 (s, 3H, CH3), 4.2 (s, 2H, CH2), 6.90 (s, 1H, pyridine H-5), 7.40-7.90 (m, 10H, Ar-H). Anal. calcd. for C25H20N6OS2 (484.6); C, 61.96; H, 4.16; N, 17.34. Found; C, 62.04; H, 4.19; N, 17.38. 7b, Yield 60%; red; mp 256- 257℃; IR (KBr) υ= 3222 (NH), 2222 (CN) and 1693 (CO) cm-1. 1H NMR (CDCl3/CF3COOD): δ/ppm = 2.4 (s, 3H, CH3), 2.5 (s, 3H, CH3), 2.6 (s, 3H, CH3), 4.2 (s, 2H, CH2), 7.0 (s, 1H, pyridine H-5), 7.2-7.9 (m, 9H, Ar-H). MS (M+; EI): m/z (%) = 498 (18). Anal. calcd. for C26H22N6OS2 (498.62): C, 62.63; H, 4.45; N, 16.85. Found: C, 62.71; H, 4.40; N, 16.74. 7c, Yield 50%; brown; mp 178- 180℃; IR (KBr) υ= 3287 (NH), 2219 (CN) and 1688 (CO) cm-1. Anal. calcd. for C26H22N6O2S2(514.62): C, 60.68; H, 4.31; N, 16.33. Found: C, 60.63; H, 4.38; N, 16.37.7d, Yield 72%; brown; mp >265℃; IR (KBr) υ= 3304 (NH), 2228 (CN) and 1698 (CO) cm-1. Anal. calcd. for C25H19N7O3S2 (529.59): C, 56.70; H, 3.62; N, 18.51. Found: C, 56.71; H, 3.67; N, 18.34. 7e, Yield 76%; brown; mp 246- 247℃; IR (KBr) υ= 3311 (NH), 2224 (CN) and 1696 (CO) cm-1. Anal. calcd. for C25H19BrN6OS2 (563.49): C, 53.29; H, 3.40; N, 14.91. Found: C, 53.21; H, 3.33; N, 14.94. Synthesis of3-amino-N-(4-phenyl-5-arylazo-2-thiazolyl)-thieno[2,3-b]pyridine-2-carboxamide dyes 8The nicotinonitrile derivative 7 (0.005 mol) was added to a solution of sodium ethoxide (from 0.005 mol sodium metal) in absolute ethanol (30 ml). The solution was refluxed for 2 hours, left to cool, and diluted with cooled water (50 ml). The solid obtained was filtered and recrystallized from ethanol. 8a, Yield 43%; red; mp >265℃; IR (KBr) υ= 3177, 3280 (NH, NH2) and 1632 (CO) cm-1. 1H NMR (CDCl3/CF3COOD): δ/ppm = 2.4 (s, 3H, CH3), 2.5 (s, 3H, CH3), 7.0 (s, 1H, pyridine H-5), 7.3-7.8 (m, 10H, Ar-H). Anal. calcd. for C25H20N6OS2 (484.6): C, 61.96; H, 4.16; N, 17.34. Found: C, 62.08; H, 4.22; N, 17.27. 8b, Yield 50%; red; mp >265℃; IR (KBr) υ= 3494, 3329, 3189 (NH, NH2) and 1636 (CO) cm-1. 1H NMR (CDCl3/CF3COOD): δ/ppm = 2.4 (s, 3H, CH3), 2.8 (s, 3H, CH3), 3.0 (s, 3H, CH3), 7.3 (s, 1H, pyridine H-5), 7.4-7.9 (m, 9H, Ar-H). MS (M+; EI): m/z (%) = 498 (23). Anal. calcd. for C26H22N6OS2 (498.62): C, 62.63; H, 4.45; N, 16.85. Found: C, 62.68; H, 4.52; N, 16.76. 8c, Yield 64%; brown; mp 264- 266℃; IR (KBr) υ= 3438, 3315 (NH, NH2) and 1636 (CO) cm-1. Anal. calcd. for C26H22N6O2S2 (514.62): C, 60.68; H, 4.31; N, 16.33. Found: C, 60.60; H, 4.40; N, 16.26. 8d, Yield 65%; brown; mp >265℃; IR (KBr) υ= 3418, 3293 (NH, NH2) and 1644 (CO) cm-1. Anal. calcd. for C25H19N7O3S2 (529.59): C, 56.70; H, 3.62; N, 18.51. Found: C, 56.72; H, 3.68; N, 18.42.8e, Yield 48%; brown; mp 254- 255℃; IR (KBr) υ= 3444, 3357, 3289 (NH, NH2) and 1638 (CO) cm-1. Anal. calcd. for C25H19BrN6OS2 (563.49): C, 53.29; H, 3.40; N, 14.91. Found: C, 53.41; H, 3.45; N, 14.97.
4.3. Dyeing of Polyester Fibers and Dyeing Properties
4.3.1. Preparation of Dye Dispersion
- Dispersion of the dye was produced by dissolving the appropriate amount of dye (0.1g dye/ 5g fiber, 2% shade) in 1 ml acetone and then added dropwise with stirring to a solution of Setamol WS (0.5 – 1.5), an anionic dispersing agent of BASF (sodium salt of a condensation product of naphthalene sulfonic acid and formaldehyde). The dyestuff dispersion was added to the dyebath at 60℃ through a fine mesh sieve or filter cloth.
4.3.2. Dyeing Procedure
- The dye bath was prepared with liquor ratio 20:1 using sealed stainless steel dye pots of 250 ml capacity in “Galvanin-Marino VI-Italy” dyeing machine. Additional dispersing agent (0.5-1.0 g/l Setamol WS of BASF) was added and the pH of the bath adjusted to 5.5 using glacial acetic acid. The polyester fibers was immersed and dyeing carried out by raising the dye bath temperature from 20 to 130℃ at a rate of 3℃ /min and holding at this temperature for 60 min before rapidly cooling to 50℃ at 9.9℃/min. The dyed fibers was then rinsed with cold water, reduction-cleared using 2 cc/l caustic soda solution 32.5% (71ºTw), 2 g/l sodium hydrosulphite and 0.5 g/l Hostapal CV conc. non-ionic wetting agent of Clariant at 75-85℃ for 15-30 minutes and soaped with 2% nonionic detergent and ammonia (pH 8.5) at 50℃ for 30 minutes to improve washing fastness then drying in a pre-drier without contact, up to a residual moisture of approximately 30% followed by final drying which be carried out on a hot flue.
4.3.3. Fastness Properties
- (i) Fastness to washing. A specimen of dyed polyester sample was stitched between two pieces of undyed cotton and polyester fabrics (10 cm 4 cm), all approximately of equal weight and then washed at 50℃. (ii) Fastness to acid and alkaline perspiration. The AATCC standard test method 15 –1960 was used. The acid solution (pH = 3.5) contained sodium chloride (10 g/l), lactic acid U.S.P 85% (1 g/l), disodium orthophosphate anhydrous (1 g/l) and histidine monohydrochloride (0.25 g/l). The alkaline solution contained sodium chloride (10 g/l), ammonium carbonate (4 g/l), disodium orthophosphate anhydrous (1 g/l) and histidine monohydrochloride (0.25 g/l).A composite specimen was made from the dyed sample as a layer between undyed cotton and polyester fabrics as the same weight as the dyed sample, composite specimen was immersed in the perspiration solution for 30 minutes with occasional agitation and squeezing to insure complete wetting, then stitched between the plastic in such a way that the dyed sample will be in a vertical position when placed in the oven. The loaded sample was kept in an oven at 37℃ for 6 – 8 hours and then dried by conventional means. (iii) Fastness to rubbing. The dyed polyester fiber was placed on the base of crockmeter, so that it rested flat on the abrasive cloth with its long dimension in the direction of rubbing. A square of white testing cloth was mount over the end of the finger which protects downward on the dry specimen sliding back and forth twenty times by making ten complete turns of the crank at the rate of one turn per second. For wet rubbing test, the testing squares were thoroughly wet in distilled water and squeezed between filter papers through hand wringer under standard conditions. The rest of the procedure is the same as the dry crocking test. (iv) Fastness to sublimation. The fastness to sublimation was assessed according to ISO/R 10S/IV – Part 2. The dyed polyester fiber was stitched between two pieces of white polyester and cotton fabrics, all of equal length. The samples were treated at 185℃and 210℃ for 30 seconds, then conditioning for 16 hours.(v) Fastness to light. The tested samples and standard blue scales were exposed to the “Weather-o-meter” (Atlas Electric Devices Co. USA) . The exposure of both was discontinued at one of the time indicated in AATCC standard of 5, 10, 20, 40, 80, 160, 320 and 640 hours, at which it shows just appreciable fading. In the present work the dyed fabrics were exposed to light for 40 hours, after which the fibers were allowed to lie in the dark at room temperature for about two hours in order to cool-off and regain normal moisture from air.
References
| [1] | A.-C. Gaumont, M. Gulea and J. Levillain, " An Overview of the Chemistry of 2-Thiazolines", Chem. Rev., vol. 109, no.3, pp. 1371-1401, 2009 |
| [2] | U.G. Ibatullin, T.F. Petrushina, L.Y. Leitis, I.Z. Minibaev and B.O. Logvin, Khim. Geterotsitl. Soedin., pp. 715, 1993 |
| [3] | P.C. Hang and J.F. Honek, " Electronic structure calculations on the thiazole-containing antibiotic thiostrepton: molecular mechanics, semi-empirical and ab initio analyses", Bioorg. Med. Chem. Lett., vol.15, no. 5, pp. 1471- 1474, 2005 |
| [4] | P. Beuchet, M. Varache-Lembège, A. Neveu, J.-M. Léger, J. Vercauteren, S. Larrouture, G. Deffieux, A. Nuhrich, " New 2-sulfonamidothiazoles substituted at C-4: synthesis of polyoxygenated aryl derivatives and in vitro evaluation of antifungal activity", Eur. J. Med. Chem., vol. 34, no. 9, pp. 773- 779, 1999 |
| [5] | A. Geronikaki, P. Vicini, N. Dabarakis, A. Lagunin, V. Poroikov, J. Dearden, H. Modarresi, M. Hewitt and G. Theophilidis, "Evaluation of the local anaesthetic activity of 3-aminobenzo[d]isothiazole derivatives using the rat sciatic nerve model", Eur. J. Med. Chem., vol. 44, no. 2, pp. 473- 481, 2009 |
| [6] | C. Papadopoulou, A. Geronikaki and D. Hadjipavlou-Litina, "Synthesis and biological evaluation of new thiazolyl/ benzothiazolyl-amides, derivatives of4-phenyl-piperazine ", II Farmaco, vol. 60, no. 11-12, pp. 969- 973, 2005 |
| [7] | A. Kreutzberger and H. Schimmelpfennig, "[Antiviral drugs, XVIII: 2-Aminothiazoles by cleavage of the S-S-bond of disulfidodicarbamidine", Arch. Pharm., vol. 314, no.5, pp. 385- 391, 1981 |
| [8] | D. Keil, R. Flaig, A. Schroeder and H. Hartmann, Dyes and Pigments, "Synthesis and characterisation of methine dyes derived fromN,N-disubstituted 2-aminoselenazoles and some of their heterocyclic sulfur analogues", vol. 50, no. 1, pp. 67- 76, 2001 |
| [9] | M.A. Metwally, E. Abdel-latif, A.M. Khalil, F.A. Amer and G. Kaupp, Dyes and Pigments, "New azodisperse dyes with thiazole ring for dyeing polyester fabrics", vol. 62, no. 2, pp. 181- 195, 2004 |
| [10] | K. Singh, S. Singh and J.A. Taylor, " Monoazo disperse dyes—part 1: synthesis, spectroscopic studies and technical evaluation of monoazo disperse dyesderived from 2-aminothiazoles", Dyes and Pigments, vol. 54, no. 3, pp. 189- 200, 2002 |
| [11] | M.S. Yen and I.J. Wang, "Synthesis and solvent characteristics of bishetaryl monoazodyes derived from polysubstituted-2-aminothiophene derivatives", Dyes and Pigments, vol. 67, no. 3, pp. 183- 188, 2005 |
| [12] | Metwally M. A., Abdel-Latif E. and Amer F. A., "New 4-arylazo-2- (substituted)-3- phenyl-1,3- thiazolidin-5-ones as disperse dyes part 1", J. Text. Assoc., Nov.- Dec., pp. 155 – 159, 2001 |
| [13] | Metwally M. A., Abdel-Latif E. and Amer F. A., "New 4-arylazo-2- (substituted)-3- phenyl-1,3- thiazolidin-5-ones as disperse dyes part 2", J. Text. Assoc., Nov.- Dec., 149- 154, 2002 |
| [14] | Metwally M. A., Etman H. A., Gafer H. E. and Khalil A. M., " New disperse dyes derived 1,3- thiazolidin-4- ones and 5- ones for dyeing polester fabrics ", Advances in Color Science and Technology, vol. 7, no. 3, pp. 71-78, 2004 |
| [15] | Metwally M. A., Abdel-Latif E., Amer F. A. and Kaupp G., "Synthesis of new 5-thiazoyl azo-disperse dyes for dyeing polyester fabrics", Dyes and Pigments, vol. 60, no. 3, pp. 249- 264, 2004 |
| [16] | Standard methods for the determination of the color fastness of textiles and leather, 5th Edn[Bradford: SDC], 1990 |
| [17] | Műller C, “Recent developments in the chemistry of disperse dyes and their intermediate”, American Dyestuff Reporter, vol. 59, no. 5, pp. 37- 44, 1970 |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML