-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Surgical Research
p-ISSN: 2332-8312 e-ISSN: 2332-8320
2020; 9(1): 1-8
doi:10.5923/j.surgery.20200901.01

Correlation between the Size of the Prostate, Post Void Residual Volume, PSA Level and IPSS in Men with LUTS in Three Major Urology Centers in Khartoum
Safaa Hussein Ibrahim Hamid 1, Ali Yasen Mohamedahmed 2, Abdel Raof Sharfi 3
1MBBS, MD, MRCSed, Sudan Medical Specialization Board
2MBBS, MSc, MD, MRCS, SWBH NHS Trust
3MD-FRCSed (Urology), Professor in Surgery Department, Faculty of Medicine, University of Khartoum
Correspondence to: Safaa Hussein Ibrahim Hamid , MBBS, MD, MRCSed, Sudan Medical Specialization Board.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Objectives: To study the prevalence of symptoms of IPSS in three major urological clinics in Khartoum and the correlation of these symptoms with PSA level, post void residual urine volume and prostate volume. Subjects and methods: This is a cross-sectional hospital based study including 100 men who presented with LUTS to three major urological centers in Sudan (Suba, IbnSina and police hospitals), in the period from November 2015 to November 2016. All patients were assessed with a well-designed questionnaire and analyzed using SPSS program. Results: The total number of patients was 100. The mean age was 69 +/- 9.6. The commonest presenting symptoms were frequency (94%) and weak stream (94%). The IPSS score was mild in (28%), moderate in (22%) and severe in (50%), most of the patients were found to be dissatisfied (27%) or mixed feelings (25%) and only 1% were not bothered with their symptoms. The mean PSA level was 6.5 +/- 6.5. The mean Prostate volume was 78.4 +/- 30.7 cc. The post void residual urine volume mean was 69.3 +/- 39.8ml. A significant (P value 0.01) direct proportion was found between IPSS total score and PSA level, prostate volume, post void residual volume and quality of life. Insignificant correlation (P value 0.911) was found between age and IPSS. A good relation was noticed between prostate volume (PV) and PSA level (P value 0.01) and prostate volume and post void residual volume (P value 0.001). Conclusion: The most common presenting symptoms were weak stream and increased frequency. A significant correlation was found between IPSS score and PSA, prostate volume, post void residual volume and quality of life. IPSS score is not age related. Large prostate was noticed to have high PSA level and post void residual urine volume.
Keywords: IPSS, Frequency, LUTS, PSA, Post void residual volume, Quality of life, Prostate volume
Cite this paper: Safaa Hussein Ibrahim Hamid , Ali Yasen Mohamedahmed , Abdel Raof Sharfi , Correlation between the Size of the Prostate, Post Void Residual Volume, PSA Level and IPSS in Men with LUTS in Three Major Urology Centers in Khartoum, International Journal of Surgical Research, Vol. 9 No. 1, 2020, pp. 1-8. doi: 10.5923/j.surgery.20200901.01.
Article Outline
1. Introduction
- The International Prostate Symptom Score (I-PSS) is based on the answers to seven questions concerning urinary symptoms and one question concerning quality of life. Each question concerning urinary symptoms allows the patient to choose one out of six answers indicating increasing severity of the particular symptom. The answers are assigned points from 0 to 5. The total score can therefore range from 0 to 35 (asymptomatic to very symptomatic).The questions refer to the following urinary symptoms:Questions Symptom:• Incomplete emptying• Frequency• Intermittency• Urgency• Weak Stream• Straining• NocturiaQuestion eight refers to the patient’s perceived quality of life. The first seven questions of the I-PSS are identical to the questions appearing on the American Urological Association (AUA) Symptom Index which currently categorizes symptoms as follows:• Mild (symptom score less than or equal to 7)• Moderate (symptom score range 8-19)• Severe (symptom score range 20-35)The International Scientific Committee (SCI), under the patronage of the World Health Organization (WHO) and the International Union against Cancer (UICC), recommends the use of only a single question to assess the quality of life. The answers to this question range from “delighted” to “terrible” or 0 to 6. Although this single question may or may not capture the global impact of benign prostatic hyperplasia (BPH) Symptoms or quality of life, it may serve as a valuable starting point for a doctor-patient conversation. The SCI has agreed to use the symptom index for BPH, which has been developed by the AUA Measurement Committee, as the official worldwide symptoms assessment tool for patients suffering from prostatism. The SCI recommends that physicians consider the following components for a basic diagnostic workup: history; physical exam; appropriate labs, such as U/A, creatine, etc.; and DRE or other evaluation to rule out prostate cancer.The justification to perform such study because is that no previous studies in Sudan looked into this problem besides that LUTS is increasing.
2. Literature Review
- Background [1]Benign prostatic hyperplasia (BPH), also known as benign prostatic hypertrophy, is a histologic diagnosis characterized by proliferation of the cellular elements of the prostate. Cellular accumulation and gland enlargement may result from epithelial and stromal proliferation, impaired preprogrammed cell death (apoptosis), or both.BPH involves the stromal and epithelial elements of the prostate arising in the periurethral and transition zones of the gland (see Pathophysiology). The hyperplasia presumably results in enlargement of the prostate that may restrict the flow of urine from the bladder.BPH is considered a normal part of the aging process in men and is hormonally dependent on testosterone and dihydrotestosterone (DHT) production. An estimated 50% of men demonstrate histopathologic BPH by age 60 years. This number increases to 90% by age 85 years.The voiding dysfunction that results from prostate gland enlargement and bladder outlet obstruction (BOO) is termed lower urinary tract symptoms (LUTS). It has also been commonly referred to as prostatism, although this term has decreased in popularity. These entities overlap; not all men with BPH have LUTS, and likewise, not all men with LUTS have BPH. Approximately half of men diagnosed with histopathologic BPH demonstrate moderate-to-severe LUTS.Clinical manifestations of LUTS include urinary frequency, urgency, nocturia (awakening at night to urinate), decreased or intermittent force of stream, or a sensation of incomplete emptying. Complications occur less commonly but may include acute urinary retention (AUR), impaired bladder emptying, the need for corrective surgery, renal failure, recurrent urinary tract infections, bladder stones, or gross hematuria.Prostate volume may increase over time in men with BPH. In addition, peak urinary flow, voided volume, and symptoms may worsen over time in men with untreated BPH. The risk of AUR and the need for corrective surgery increases with age.Patients who are not bothered by their symptoms and are not experiencing complications of BPH should be managed with a strategy of watchful waiting. Patients with mild LUTS can be treated initially with medical therapy. Transurethral resection of the prostate (TURP) is considered the criterion standard for relieving bladder outlet obstruction (BOO) secondary to BPH. However, there is considerable interest in the development of minimally invasive therapies to accomplish the goal of TURP while avoiding its adverse effects.Anatomy [1]:The prostate is a walnut-sized gland that forms part of the male reproductive system. It is located anterior to the rectum and just distal to the urinary bladder. It is in continuum with the urinary tract and connects directly with the penile urethra. It is therefore a conduit between the bladder and the urethra. The gland is composed of several zones or lobes that are enclosed by an outer layer of tissue (capsule). These include the peripheral, central, anterior fibromuscular stroma, and transition zones. BPH originates in the transition zone, which surrounds the urethra.Pathophysiology [1]:Prostatic enlargement depends on the potent androgen dihydrotestosterone (DHT). In the prostate gland, type II 5-alpha-reductase metabolizes circulating testosterone into DHT, which works locally, not systemically. DHT binds to androgen receptors in the cell nuclei; potentially resulting in BPH.In vitro studies have shown that large numbers of alpha-1-adrenergic receptors are located in the smooth muscle of the stroma and capsule of the prostate, as well as in the bladder neck. Stimulation of these receptors causes an increase in smooth-muscle tone, which can worsen LUTS. Conversely, blockade of these receptors (see Treatment and Management) can reversibly relax these muscles, with subsequent relief of LUTS.Microscopically, BPH is characterized as a hyperplastic process. The hyperplasia results in enlargement of the prostate that may restrict the flow of urine from the bladder, resulting in clinical manifestations of BPH. The prostate enlarges with age in a hormonally dependent manner. Notably, castrated males (ie, who are unable to make testosterone) do not develop BPH. The traditional theory behind BPH is that, as the prostate enlarges, the surrounding capsule prevents it from radially expanding, potentially resulting in urethral compression. However, obstruction-induced bladder dysfunction contributes significantly to LUTS. The bladder wall becomes thickened, trabeculated, and irritable when it is forced to hypertrophy and increase its own contractile force. This increased sensitivity (detrusor overactivity [DO]), even with small volumes of urine in the bladder, is believed to contribute to urinary frequency and LUTS. The bladder may gradually weaken and lose the ability to empty completely, leading to increased residual urine volume and, possibly, acute or chronic urinary retention. In the bladder, obstruction leads to smooth-muscle-cell hypertrophy. Biopsy specimens of trabeculated bladders demonstrate evidence of scarce smooth-muscle fibers with an increase in collagen. The collagen fibers limit compliance, leading to higher bladder pressures upon filling. In addition, their presence limits shortening of adjacent smooth muscle cells, leading to impaired emptying and the development of residual urine. The main function of the prostate gland is to secrete an alkaline fluid that comprises approximately 70% of the seminal volume. The secretions produce lubrication and nutrition for the sperm. The alkaline fluid in the ejaculate results in liquefaction of the seminal plug and helps to neutralize the acidic vaginal environment. The prostatic urethra is a conduit for semen and prevents retrograde ejaculation (ie, ejaculation resulting in semen being forced backwards into the bladder) by closing off the bladder neck during sexual climax. Ejaculation involves a coordinated contraction of many different components, including the smooth muscles of the seminal vesicles, vasa deferentia, ejaculatory ducts, and the ischiocavernosus and bulbocavernosus muscles.The diagnosis of benign prostatic hyperplasia (BPH) can often be suggested on the basis of the history alone. Special attention to the following features is essential to making the correct diagnosis:• Onset and duration of symptoms• General health issues (including sexual history)• Fitness for any possible surgical interventions• Severity of symptoms and how they are affecting quality of life• Medications• Previously attempted treatmentsSymptoms often attributed to BPH can be caused by other disease processes, and a history and physical examination are essential in ruling out other etiologies of (lower urinary tract symptoms (LUTS). When the prostate enlarges, it may act like a "clamp on a hose," constricting the flow of urine. Nerves within the prostate and bladder may also play a role in causing the following common symptoms:• Urinary frequency - The need to urinate frequently during the day or night (nocturia), usually voiding only small amounts of urine with each episode• Urinary urgency - The sudden, urgent need to urinate, owing to the sensation of imminent loss of urine without control• Hesitancy - Difficulty initiating the urinary stream; interrupted, weak stream• Incomplete bladder emptying - The feeling of persistent residual urine, regardless of the frequency of urination• Straining - The need strain or push (Valsalva maneuver) to initiate and maintain urination in order to more fully evacuate the bladder• Decreased force of stream - The subjective loss of force of the urinary stream over time• Dribbling - The loss of small amounts of urine due to a poor urinary streamA sexual history is important, as epidemiologic studies have identified LUTS as an independent risk factor for erectile dysfunction and ejaculatory dysfunction [2].The American Urological Association (AUA) has developed rigorous clinical practice guidelines for BPH [3] The AUA guidelines were based on the 1994 evidence-based guidelines for the diagnosis and treatment of BPH originally created under the auspices of the United States Department of Health and Human Services Agency for Health Care Policy and Research. [4] The AUA updated its guidelines in 2006 and 2010, and reviewed and confirmed their validity in 2014. [3]
3. Recommended Tests
- A medical history should be taken to qualify and quantify voiding dysfunction. Identification of other causes of voiding dysfunction and medical comorbidities are essential to properly assess the condition and to determine conditions that may complicate treatment.The physical examination consists of a focused physical examination and a neurologic examination. The physical examination includes a DRE to measure prostate size and to assess for abnormalities. The neurological examination is geared toward lower-extremity neurologic and muscular function, as well as anal sphincter tone. Examination of the phallus and foreskin occasionally reveals meatal stenosis, unretractable foreskin, penile ulcers, or foreign bodies such as warts.PSA testing should be offered to any patient with a 10-year life expectancy in whom the diagnosis of prostate cancer would change management. The severity of BPH can be determined with the International Prostate Symptom Score (IPSS)/American Urological Association Symptom Index (AUA-SI) plus a disease-specific quality of life (QOL) question. The AUA-SI for BPH is a set of 7 questions that has been adopted worldwide and yields reproducible and quantifiable information regarding symptoms and response to treatment. Questions concern incomplete emptying, frequency, intermittency, urgency, weak stream, straining, and nocturia.The IPSS uses the same 7 questions as the AUA-SI, with the addition of an eighth question, known as the bother score, which is designed to assess perceived disease-specific QOL. The AUA-SI/IPSS questionnaire is available online. Based on the sum of the score for all 8 questions, patients are classified as 0-7 (mildly symptomatic), 8-19 (moderately symptomatic), or 20-35 (severely symptomatic).]The AUA 2010 guideline update lowered the age of the Index Patient from age 50 years or older to age 45 years or older. Two algorithms were published: the algorithm for diagnosis and basic management of LUTS in the Approach section above, and an algorithm for detailed management of bothersome LUTS that persists after basic management.Patients with mild symptoms (IPSS/AUA-SI score <7) or moderate-to-severe symptoms (IPSS/AUA-SI score ≥8) of benign prostatic hyperplasia (BPH) who are not bothered by their symptoms and are not experiencing complications of BPH should be managed with a strategy of watchful waiting. In these situations, medical therapy is not likely to improve their symptoms and/or quality of life (QOL). In addition, the risks of treatment may outweigh any benefits. Patients managed expectantly with watchful waiting are usually re-examined annually.Transurethral resection of the prostate (TURP) has long been accepted as the criterion standard for relieving bladder outlet obstruction (BOO) secondary to BPH. In current clinical practice, most patients with BPH do not present with obvious surgical indications; instead, they often have milder lower urinary tract symptoms (LUTS) and, therefore, are initially treated with medical therapy. The era of medical therapy for BPH dawned in the mid-1970s with the use of nonselective alpha-blockers such as phenoxybenzamine. The medical therapeutic options for BPH have evolved significantly over the last 3 decades, giving rise to the receptor-specific alpha-blockers that comprise the first line of therapy.In a study conducted by J.L.H.R. Bosch et al. [5] a community-based population of 502 men aged between 55 and 74 years with no prostate Cancer and no history of a prostate operation; Results Overall, 6 and 24% of the men were severely and moderately symptomatic, respectively. The results of a detailed questionnaire such as the IPSS (only 12% of the men scored 0) contrast with the men's global perception of their voiding function (82% claimed to have ‘no voiding complaints’). A good correlation was found between the total symptom score and the single disease-specific quality of life question that is included in the IPSS (r= 0.74, P= 0.001). There was a weak correlation between the IPSS and total prostate volume (r= 0.19, P<0.001), and between the IPSS and physiological measures such as peak flow rate (r=— 0.18, P<0.001) and post-void residual urine volume (r= 0.25, P<0.001). There was a very weak correlation between the IPSS and age (r=0.09, P= 0.04).Stepan Veselya et al. [6] reported that A series of 354 men (mean age 70.2 years; range 45-91 years) with LUTS due to BPE were stratified into seven age groups and reviewed retrospectively. All patients underwent a standard evaluation, involving determination of the International Prostate Symptom Score (IPSS), digital rectal examination, uroflowmetry, determination of the prostate-specific antigen (PSA) level and transrectal ultrasonography. Descriptive statistics were used to describe all the variables and Spearman's correlation test was used to evaluate the relationships between them. Results: The mean prostate volume was 40.1 (±23.9) cm and mean PSA concentration 3.9 (±4.2) ng/ml. Both values increased progressively from 27.5 ml and 1.5 ng/ml, respectively in the <54 years age group to 48.2 ml and 5.4 ng/ml, respectively in the <80 years age group. However, in the 75-79 years age group there was a decrease in both prostate volume and symptom score; PSA concentration remained unchanged and maximal flow rate increased slightly. A statistically significant but weak correlation was found between prostate volume and age (r = 0.25, p < 0.0001) and between PSA and age (r = 0.28, p < 0.0001). Prostate volume correlated positively with serum PSA (r = 0.54, p < 0.0001). The correlations between maximum flow rate and age, prostate volume, PSA and IPSS were r = −0.21, p < 0.0001; r = −0.18, p < 0.0006; r = −0.29, p < 0.0001; and r = 0.14, p < 0.0098, respectively. Hans Hedelina et al [7] reported that BPH was estimated to be the main etiological agent in less than every second man. There was a statistically significant correlation between s-PSA and prostate size. However, among men with s-PSA<1.5 ng/ml, one-third had a prostate volume of >30 ml and 17% a prostate volume of >40 ml. Among men with s-PSA≥1.5 ng/ml, as many as 18% had a prostate volume of ≤30 ml and 42% a prostate volume of ≤40 ml. Bladder and/or prostate cancer was diagnosed in 8% of men, mostly as a coincidental finding. Taiji Tsukamoto et al. [8] recorded that of all BPH patients who attended the Urology Clinic of Sapporo Medical University Hospital, during December 2003 and June 2004 with the inclusion criterion that they have at least two PV and lower urinary tract symptoms measurements using the International Prostate Symptom Score (IPSS). Sixty-seven patients were eligible. Baseline PV correlated with residual urine volume (r = 0.37, P < 0.05) and prostate-specific antigen (PSA; r = 0.65, P < 0.001) but not IPSS (r =−0.16). PV increased in 46 (70%) men, remained the same in 10 and decreased in 11; in the former group, the mean prostate enlargement generally increased as baseline PV increased. In multiple linear regression models that included baseline IPSS, correlation between change in IPSS and change in PV was 0.47 (P = 0.05) based on 25 patients with measures at concurrent visits. Change in PV was also correlated with change in quality of life score (0.46, P = 0.02) but not with change in PSA (r = 0.38, P = 0.07, maximum flow rate (−0.24) or residual urine volume (−0.06). Masumori N et al. [9] evaluated the International Prostate Symptom Score (IPSS) and quality-of-life (QOL) score of 235 outpatients having lower urinary tract symptoms and 242 participants in a community-based study of Japanese men aged 50 to 79 years old.Although the proportion of outpatients in the severe IPSS category (IPSS 20 to 35) was greater than that in the participants of the community-based study in each age decade, the proportion in the moderate IPSS category (IPSS 8 to 19) in both groups overlapped each other. On the other hand, the distribution of QOL scores was considerably different, with only a small portion of overlap in each age decade. Although scores for both voiding symptoms (incomplete emptying, intermittency, weak stream, and hesitancy) and storage symptoms (increased frequency, urgency, and nocturia) were significantly greater in outpatients than in study participants in each age decade, the difference was more obvious for voiding symptoms than for storage symptoms.
4. Patients and Methods
- The study was a prospective cross sectional hospital based study. The study area was multicenter including three major urological centers in Khartoum (Soba university hospital, Ibn Sina teaching hospital and Ribat university hospital). Study population included all men presenting with LUTS to these three major urological centers in Khartoum. The duration of the study was the period from November 2015 to November 2016. The sample size was100 patients included all men presenting with LUTS and excluded patients who refused to participate in the study, or did not complete the requested investigations and patients with UTI or vesical stones. the data was collected with a predesigned questionnaire. Data variables: IPSS symptoms, prostate volume which was assessed by abdominal U/S and performed by the radiologist and measured in 3D in CC, post void residual volume which was also measured by abdominal U/S and PSA. the data was analyzed using SPSS computer program version 25 and a verbal consent was taken from all patients after explaining the study.
5. Results
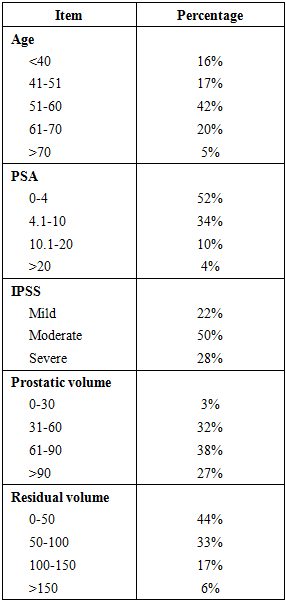
- The number of patients who were included in this study in the period between January 2015 and December 2016 was 100 patients. Their age ranged between 50 and 95 years with a mean of 69± 9.6 years. The commonest age was between 66 and 75 years. Age and PSA level were found related significantly (P value 0.0001) but there was no correlation (P value 0.911) between age and IPSS.In this study the frequency of prostatic symptoms according to IPSS score was as follows: 81% had incomplete empting of the urinary bladder, 91% had urgency, 94% had increased frequency, 82% had intermittency 94% had week stream, 77% had nocturia, while 49% had straining.Fifty percent of the patients were found to have moderate IPSS score (8-19) whereas 22% and 28% were found to have mild (1-7) and severe (20-35) respectively.Regarding the quality of life, the majority of the interviewed patients had mixed symptoms (25%) and mostly dissatisfied (27%). 15 patients were mostly satisfied and 17% were unhappy with their symptoms. However 13% patients had terrible quality of life and only 1% patient was delighted and 2% were pleased with their symptoms.The mean PSA level was 6.5±5.6 ng/dl. fifty two percent had PSA level ranging from (0-4ng/dl), 34% had PSA level (4-10ng/dl), 10% had PSA level (10-20ng/dl), while only 4% had PSA (>20ng/dl).Ultra Songraphic measurement of the prostate revealed a mean volume of 78.4±30.7 CC. 32 patients had prostate volume (PV) ranged between 31 and 60 CC, 38 patients had PV between 61 and 90 CC, 27 patients had more than 90 CC PV and only in 3 patients it measured less than 30 CC. A significant relation was noticed between prostate volume (PV) and PSA level in a direct proportion (P value 0.001).The mean post void residual urine volume (PVR) was 69.3 +/- 39.8 ml. 44 patients showed residual volume less than 50 ml. 33 patients had PVR between 51 to 100 and 23 patients had volume more than 100 ml. The prostate volume (PV) is associated with elevated post void residual volume (PVR) significantly (P value 0.001). In this study a significant direct proportion was found between IPSS total score and PSA level, prostate volume, post void residual volume and the quality of life, (P value 0.001).
|
6. Discussion
- Lower urinary tract symptoms (LUTS) represent one of the most common clinical complaints in adult men [10]. The prevalence of LUTS increases with age, and estimates vary widely depending on definitions and cohorts studied [10,11]. LUTS have a major impact on health-related quality of life (QOL) [11] and are associated with substantial personal and social costs [12]. This is prospective cross sectional hospital based study included 100 men presenting with LUTS to three major urological centers in khartoum state (Suba, Ibn Sina and Ribat university hospitals), in the period from November 2015 to November 2016. The age distribution of the contributed patients was between 50 to 95 with mean of 69 +/- 9.6 years, the commonest age group was 66-75. Interestingly no correlation (P value 0.911) was found between age and IPSS. On the other hand, another study [30] showed that the specific urinary symptoms of LUTS are strongly age-related and, therefore, may be predictive of a prostatic disease process.Fifty percent of the patients were found to have moderate IPSS score (8-19), whereas 28% were classified as severe (20-35) and 22% as mild (0-7). The cumulative frequency plot of the total symptom score of Bosch et al [5] shows that the percentages of men with minor, moderate and severe symptoms were 70%, 24% and 6%, respectively but his study was community based study unlike this study which was hospital based one so it was expected to have higher readings [9]. In other community based study which was conducted in Seoul [11] among 1360 men, 83.8%, 12.5% and 3.7% respectively, ranked between IPSS score of 1 and 7, 8 and 19, and 20 or more. The majority of the interviewed patients were found to have mixed symptoms (25%) and mostly dissatisfied (27%). 15 patients said they were mostly satisfied and 17% ones admitted they were unhappy with their symptoms. However 13% patients said they had terrible quality of life and only 1% patient was delighted and 2% were pleased with their symptoms. Compared to another study [5] which showed that 31% of men were ‘delighted’, 24% were ‘pleased’, 29% ‘mostly satisfied’ and 10% felt ‘about equally satisfied and dissatisfied’ about their urinary condition. Few men were ‘mostly dissatisfied’ (5%) or felt ‘unhappy’ (1%). No man scored 6, i.e. ‘terrible’.In another study which was held by WHO [12] the participants were divided into two groups, according to the IPSS score results: group I: Scores 8-35 (moderate/severe symptoms) and group II: Scores 0-7 (absence/mild symptoms) and concluded that men aged 65-years or over with moderate/severe LUTS have worse QOL ratings for almost all evaluation parameters proposed by the World Health Organization, according to the WHO instruments, especially social and environmental relationships, compared with mildly symptomatic or asymptomatic men in the same age group.A good correlation was found between total symptoms score and the quality of life single question (p value <0.001). Many studies supported this result [5,12].In discussion of the value of measuring PSA level, the mean was 6.5 +/- 6.5 ng/dl, 52% of the patients were found to have PSA level below 4 ng/dl whereas 34% between 4.1-10 ng/ml, 10% of them between 10.1-20ng/ml and only 4% has PSA level more than 20ng/ml.).Battikhi et al [13] aimed to find out age-specific values and ranges of total PSA (TPSA) and free PSA (FPSA) in patients with prostatism symptoms to ensure low false-positive biopsy rates. Because of the greater variability at older age, the following age-specific reference ranges of TPSA and FPSA for patients with prostatism symptoms were as follows: 3.1 and 0.7 ng/ml for the age group 40–49 years, 4.4 and 0.89 ng/ml for the age group 50–59 years, 5.6 and 1.3 ng/ml for the age group 60–69 years, and 6.3 and 1.8 ng/ml for age group 70–79 years. There was a continuous increase in TPSA and FPSA means and medians with significant correlation (P < 0.001, P < 0.005) and advancing age group.A significant relation was found between age and PSA level, this is in agreement with some researches which confirmed that PSA level readings increased with age [13]. Also PSA testing is used as a screening tool, and as a predictor of future risk for prostatic cancer [13], which was addressed by 2013 Prostate Cancer World Congress in Melbourne and have generated a set of consensus statements regarding the use of PSA testing [14]. The goal of these statements is to bring some clarity to the confusion that exists with existing guidelines, and to present reasonable and rational guidance for the early detection of prostate cancer today [15]. Moreover, another studies showed probability of an abnormal screening result of PSA based on age, race, and prostate-specific antigen threshold [16,17,18].Hammerer et.al [19] analyzed the correlation between serum prostate specific antigen (PSA) levels and the volume of the individual glandular zones of the human prostate, transition zone volume showed the greatest variation because of BPH. The mean average ratio of peripheral zone volume to central zone volume was nearly 3:1. These data strongly support the concept of age-adjusted PSA levels [13], since most of the increase in size of the prostate with increasing patient age comes from the transition zone from which BPH develops.Regarding prostate volume (PV), the mean was 78.4 +/- 30.7 cc. Only 3 patients had prostate volume less than 30 cc. 32 had PV 31-60 cc. 38 found to have 61-90 and 27 patients had more than 90 cc. In other study [20] the size of the total prostate was found 42.5cc; however it was difficult to find a correlation between prostate volume and the total IPSS score. In the contrary to this study which showed a significant positive relation between them (P value <0.001) and in addition to prostate volume and PSA level. Many other studies confirmed the good relation between prostate volume, IPSS score and PSA level [21]. Tang P et al [22] revealed that PV was the strongest predictor of prostatic cancer risk (odds ratio, 0.02; P<0.001) compared to other variables in men with PSA measuring 10–50 ng ml−1. As the prostatic cancer rates in men with PVs measuring <60 and ≥60 ml in the 10–19.9 ng/ ml PSA group were 40.6% and 15.1%, respectively, while the rates for men with PSAs measuring 20–50 ng/ ml were 65.1% and 26.8 [23].The mean post void residual urine volume (PVR) was 69.3 +/- 39.8 ml. 44% patients showed PVR less than 50 ml, 33% patients had PVR between 51 to 100 and 23% patients had volume more than 100 ml. Ultrasound (US) bladder volume measurement is generally the preferred approach for measuring PVR [24], which is not necessarily associated with BOO, since high PVR can be a consequence of BOO and/or poor detrusor function (under activity) [25,26]. High baseline PVR was associated with an increased risk of symptoms deterioration [27]. In addition, monitoring of PVR changes over time could predict AUR occurrence; patients who subsequently developed AUR showed a steady increase in PVR [27]. Kolman et.al [28] found a significant correlation between PVR and prostate volume (rs = 0.24, p <0.001). The odds of post-void residual volume greater than 50 ml were 2.5 times greater for men with prostate volume greater than 30 ml than those with smaller prostates. In regression analyses [28], post-void residual volume did not appear to be associated with the American Urological Association symptom index, age or peak urinary flow rate, men with enlarged prostate volume or post-void residual greater than 50 ml. At baseline were about 3 times as likely to have subsequent acute urinary retention with catheterization during 3 to 4 years of follow up. However, in this study it showed a positive significant correlation between post void residual volume and both IPSS and prostate volume. Large PVR yielded a significant 2-fold up to a 4-fold increased risk of invasive therapy compared to small PVR in both treatment groups. In multivariate models these significant risk differences largely disappear, although a statistically not significant higher risk remains for the large PVR (greater than 300 ml) patients [29]. Regarding IPSS symptoms: the commonest ones were frequency and weak stream (94% each), followed by urgency (91%), intermittency (82%), incomplete emptying (81%), nocturia (77%) and lastly straining (49%). In a comparison with other community based study which was done in Swedish [31] that showed the most prevalent symptoms were urgency (69%), nocturia (60%), urge incontinence (35%), hesitancy (61%) and staining (36). A local study conducted by Eldwo et al [32] showed almost near percentages
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML