-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Surgical Research
p-ISSN: 2332-8312 e-ISSN: 2332-8320
2017; 6(1): 5-17
doi:10.5923/j.surgery.20170601.02

The Impact of HMG-CoA Reductase Inhibitors on Neutrophil Function in Patients Undergoing Colorectal Cancer Resection: An In-vitro Experimental Study
J. J. R. Richardson1, 2, C. Hendrickse2, F. Gao-Smith1, D. R. Thickett1
1Institute of Inflammation and Ageing, College of Medical and Dental Sciences, The University of Birmingham, United Kingdom
2Department of Colorectal Surgery, Heart of England NHS Foundation Trust, Birmingham, United Kingdom
Correspondence to: J. J. R. Richardson, Institute of Inflammation and Ageing, College of Medical and Dental Sciences, The University of Birmingham, United Kingdom.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

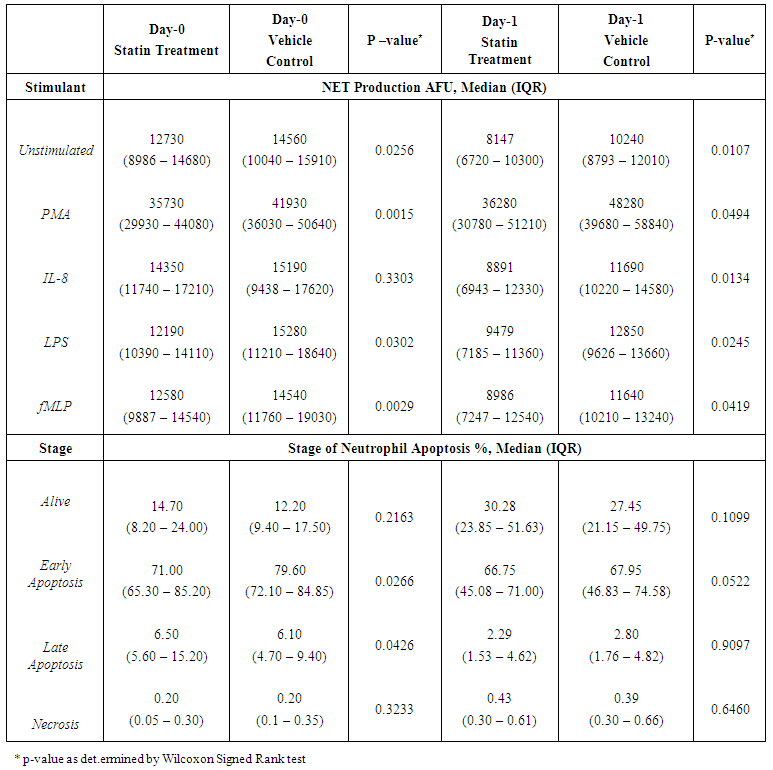
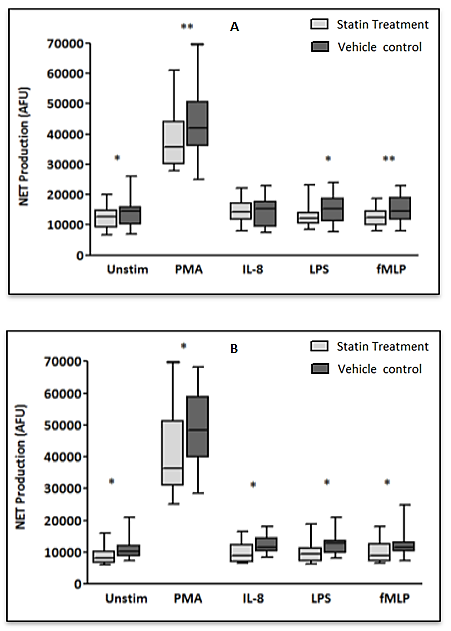
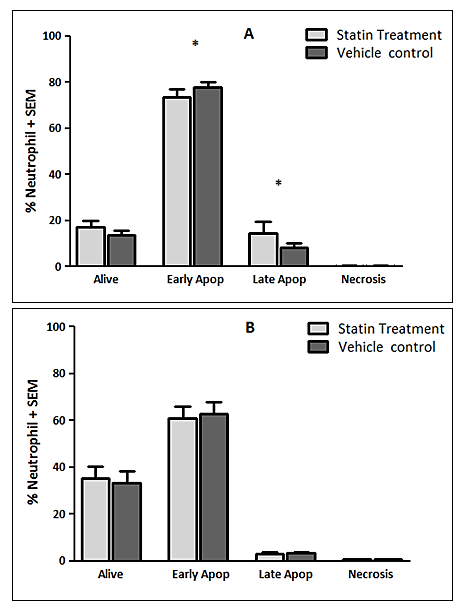
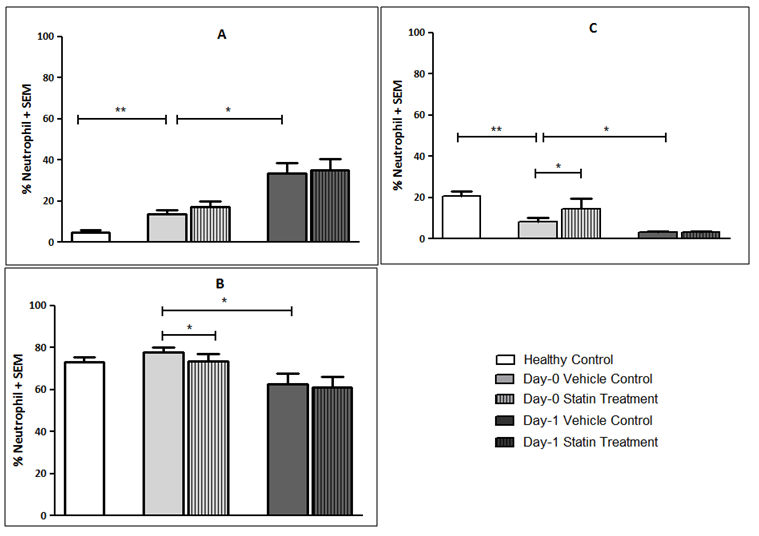
Background: Peri-operative immune-modulatory therapies aim to suppress non-specific systemic inflammation and maintain an effective anti-tumour, cell-mediated immunity of the host. Neutrophils are appreciated to have an important role in cancer progression and dissemination. The neutrophil functional defects observed in patients with colorectal cancer and as a consequence of the surgical insult are explored and the impact of HMG-CoA Reductase Inhibitors (statins) on neutrophil function is investigated in-vitro. Materials and Methods: Systemic neutrophils were isolated from healthy individuals (n=15) and from patients with colorectal cancer (n=15) who underwent surgical resection on Day-0 (pre-operatively) and Day-1 (post-operatively). Neutrophils isolated from patients were treated with 1μM simvastatin in-vitro. Neutrophils were stimulated to produce Neutrophil Extra-cellular Traps (NETs) which were quantified by a measure of the fluorescence of the extra-cellular DNA and neutrophil apoptosis was measured by fluorescence activated cell sorting. Results: Significant increases in NET production in response to No stimulant (p=0.0039), IL-8 (p=0.0039) and LPS (p=0.0157) and significant changes indicating impaired neutrophil apoptosis (p<0.001) were identified in patients with colorectal cancer when compared to healthy individuals. Following surgical resection, a significant reduction in NET production in response to fMLP (p=0.0353) and significant changes demonstrating impaired neutrophil apoptosis (p<0.010) were identified. Significant reductions in NET production were demonstrated in patients treated with 1μM simvastatin in-vitro on Day-0 (No stimulant [p=0.0256], PMA [p=0.0015], LPS [p=0.0302], fMLP [p=0.0029]) and Day-1 (No stimulant [p=0.0107], PMA [p=0.0494], IL-8 [p=0.0134], LPS [p=0.0245], fMLP [p=0.0419]). Significant reductions in early apoptotic neutrophils (71.0% vs. 79.6%, p=0.0266) and significant increases in late apoptotic neutrophils (6.5% vs. 6.1%, p=0.0426) were observed in patients treated with 1μM simvastatin in-vitro on Day-0 indicating a restoration of impaired apoptosis.' Conclusions: A therapeutic strategy aimed at modifying tumour-neutrophil interactions may maximise the therapeutic benefit of surgery. Statins are promising immune-modulatory agents in the context of cancer surgery.
Keywords: Colorectal Cancer, HMG-CoA Reductase Inhibitors, Neutrophil, Neutrophil Extracellular Traps, Statins, Surgery
Cite this paper: J. J. R. Richardson, C. Hendrickse, F. Gao-Smith, D. R. Thickett, The Impact of HMG-CoA Reductase Inhibitors on Neutrophil Function in Patients Undergoing Colorectal Cancer Resection: An In-vitro Experimental Study, International Journal of Surgical Research, Vol. 6 No. 1, 2017, pp. 5-17. doi: 10.5923/j.surgery.20170601.02.
Article Outline
1. Introduction
- Cancer surgery elicits a high-grade, non-specific systemic inflammatory response (SIR). This overwhelming, systemic inflammation suppresses systemic cell-mediated immunity and consequently compromises the tumour immunity in the host [1, 2]. This has been described as the ‘immune-hit’ [1]. The immune-hit can be exacerbated by pre-operative SIR, for example, an emergency presentation with colonic obstruction, bleeding or perforation, confers a higher risk of disease recurrence independent of the stage of disease [3]. Additionally, the development of post-operative complications and the associated post-operative SIR increases the risk of disease recurrence [4] and is associated with poor oncological outcomes and increased mortality secondary to metastatic disease [5-9]. Peri-operative immune-modulatory therapies aim to suppress non-specific systemic inflammation and maintain an effective anti-tumour, cell-mediated immunity of the host which may assist the clearance of circulating tumour cells and the development of occult metastases [1]. Accumulating evidence suggests that simple immune-modulatory strategies (anti-inflammatories [non-steroidal anti-inflammatory drugs] or immune-modulatory therapies [corticosteroids, HMG-CoA reductase inhibitors (statins)]) could be safely implemented in the peri-operative period, although concerns surrounding their side effect profile exist [10]. Statins have been extensively studied and have proven efficacy in the primary and secondary prevention of cardiovascular morbidity and mortality in a variety of populations [11-14]. Their main mechanism of action is competitive inhibition of hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase leading to a reduction in endogenous cholesterol biosynthesis and a reduction in low-density lipoprotein which is a major risk factor for cardiovascular disease [15, 16]. The cholesterol independent effects of statins are known as pleiotropic effects and they have been shown to modulate the innate and adaptive immune systems and have anti-inflammatory effects which counteract the detrimental effects of inflammation [17, 18]. In the context of colorectal surgery, statin use is associated with a significantly lower incidence of SIR and a lower incidence of surgical site infection (SSI) [19]. There appears to be no difference in overall mortality, total complications or median hospital length of stay between statin users and non-statin users undergoing major colorectal resection [20, 21], despite statin users having a greater baseline peri-operative risk compared to non-statin users, and it has been proposed that peri-operative statin therapy may reduce morbidity after colorectal resection [19, 22]. Neutrophils function as the first-line of defence against infections and are responsible for the containment and elimination of pathogens. They are prevalent at sites of tissue trauma and are the hallmark of acute inflammation [23]. In health, circulating neutrophils adhere to the endothelium before transmigrating through it in a process termed chemotaxis. At the site of inflammation neutrophils kill invading pathogens by phagocytosis, extracellular reactive oxygen species (ROS) release and by producing neutrophil extracellular traps (NETs), which constitute a DNA backbone containing histones and neutrophil granule products [24]. Neutrophils subsequently undergo apoptosis which is an important process that contributes to the resolution of the inflammation [25]. Importantly, the process of oxidant dependent NET formation also results in eventual cell death which is distinct from apoptosis [26, 27] and this allows neutrophils to exert their anti-microbial functions beyond their typical lifespan [24].There is increasing evidence that statins are able to modulate neutrophil function. In sepsis it has been demonstrated that statins reduce the neutrophil infiltrate following lipopolysaccharide (LPS) challenge in bronchoalveolar lavage fluid taken from patients receiving simvastatin [28]. In addition, the reduction in neutrophil counts was not associated with a decrease in pro-inflammatory mediator expression, which suggests a reduced neutrophil response or increased neutrophil apoptosis [28]. Statins have also been shown to increase NET formation in healthy individuals with the consequent elimination of bacteria in-vitro [29]. Furthermore, during bacterial pneumonia associated sepsis, NET production is suppressed with improvements occurring during sepsis resolution [30]. It has been proposed that neutrophil functional defects may be restored by the administration of statins [30].This is particularly relevant in the context of cancer surgery as neutrophils are appreciated to have an important role in cancer progression and dissemination [31]. Neutrophils play a vital role in circulating tumour cell metastases. This has been shown in-vitro and in-vivo where neutrophils facilitate circulating tumour cell adhesion to both pulmonary and hepatic endothelial surfaces [31-34]. The direct contact of circulating tumour cells and neutrophils is an important precursor to the development of metastatic disease [33, 34]. Following dissemination, tumour cells must be able to proliferate to form stable metastatic foci. NETs have been implicated in a direct proliferative role and have also been demonstrated to inhibit tumour cell apoptosis [31]. The ability of tumours to predispose neutrophils to produce NETs has been demonstrated and various tumour types have predisposed circulating neutrophils to produce NETs [35]. The evidence supports the theory that primary tumours can facilitate NET production in circulating neutrophils and we propose that neutrophil functional defects in cancer may be modulated by statin therapy. It is anticipated that in-vitro treatment with statin will attenuate the neutrophil functional changes observed in patients with a diagnosis of colorectal cancer and modulate the neutrophil functional changes exhibited as a consequence of the surgical insult. This may help to explain the observed benefits associated with statin therapy in the context of surgery for colorectal cancer.
2. Methods
- The study was conducted in accordance with the ethical principles and was approved by the NRES Committee, West Midlands on 30th December 2013 (13/WM/0485).
2.1. Recruitment of Patients
- Patients undergoing an elective colorectal resection for cancer, within an established enhanced recovery programme, were identified at the Colorectal Cancer Multi-disciplinary Team (MDT) meeting. All patients over the age of 18 who were undergoing elective colorectal resection for cancer and were able to give written informed consent were eligible to be included in the study. Patients were excluded from the study if they were an acute presentation, pregnant or breast feeding or if consent was refused. All patients with a prior diagnosis of inflammatory bowel disease (Ulcerative Colitis, Crohn’s Disease), synchronous or metachronous malignancy or patents receiving any known immune-modulatory therapies (suppression immune-modulatory therapies; corticosteroids, monoclonal antibodies and activation immune-modulatory therapies; granulocyte-colony stimulating factor [G-CSF]) were also excluded from the study.Patient Demographics, Patient Co-morbidities, Pre-operative Risk Prediction, Tumour Characteristics and Operative Characteristics were recorded from study participants.
2.2. Recruitment of Healthy Controls
- Healthy volunteers were required to act as controls for patients. They were recruited from the Birmingham 1,000 Elders Cohort, a research cohort of healthy volunteers above the age of 60 willing to take part in medical research. To be considered healthy, participants were not suffering from serious debilitating acute or chronic illness. Well controlled health problems, such as hypertension and asthma did not preclude enrolment.
2.3. Sample Collection
- Patients and Healthy Controls underwent peripheral blood tests performed by an experienced medical practitioner by peripheral venepuncture.
2.4. Neutrophil Isolation
- 4mls of 2% Dextran (Sigma-Aldrich, Dorset, UK) was added to 24mls of blood and gently mixed by inversion. The solution was incubated at room temperature for 30 minutes to sediment the erythrocytes. The leucocyte-rich plasma was carefully layered on a Percoll (Sigma-Aldrich) density gradient consisting of 2.5mls of 80% Percoll and 5mls of 56% Percoll in a 15ml sterile Falcon-TM tube. This was then centrifuged at 220G for 20 minutes at room temperature. The neutrophils were then isolated from the 80% and 56% gradient interface.The neutrophils were suspended and subsequently washed in Phosphate Buffered Saline (PBS; Gibco Invitrogen, Paisley, UK). The resultant supernatant was removed and the neutrophils re-suspended in RPMI-1640 (Sigma-Aldrich) at a concentration of 1x106/ml.The purity and the viability of the neutrophil yield were checked using Giemsa stain (Diff-Qik, Gentaur Europe, Brussels, Belgium) and Tryptan-Blue exclusion respectively. A purity of ≥95% and a viability of ≥97% were routinely achieved.
2.5. Quantification of Neutrophil Extracellular Trap Formation
- Neutrophils were re-suspended in RPMI-1640 with 2nM L-Glutamine, 100U/ml Streptomycin and 100ug/ml Penicillin (all from Sigma-Aldrich) at a concentration of 1x106/ml. 1x105 neutrophils, at a concentration of 1x106/ml, were sited in 20 wells of a 96-well flat bottomed plate (Becton-Dickinson). An additional 75μl of RPMI-1640 with GPS was added to each well. 25μl of RPMI-1640 with GPS and 25μl Phorbol-12-myristate-13-acetate (PMA) (1mg/ml [1:800 then 1:10 dilution]) were each added to four wells as negative and positive controls respectively. Additionally, 25μl of the following stimulants (all from Sigma-Aldrich) were each added to four wells: interleukin-8 (IL-8) (6.25μM [1:625 dilution]), Lipopolysaccharide (LPS) (1mg/ml [1:1250 dilution]) and N-formylmethionyl-leucyl-phenylalanine (fMLP) (10mM [1:500 dilution]). The plate was then incubated for 3 hours at 37°C with 5% CO2. Each well was then treated with 200units of Micrococcal Nuclease (MNase; Sigma-Aldrich) and 1μM of SYTOX-Green (Gibco Invitrogen) and incubated for 10 minutes at room temperature in the dark. This process stained and digested the extra-cellular DNA. The contents of each of the wells were then transferred into individual 500μl eppendorfs and pelleted at 5000rpm for 10 minutes. 160μl of the DNA containing supernatant was transferred into a black 96-well flat bottomed plate (CoStar, Sigma-Aldrich) and fluorescence measured in a BioTek-Synergy-2 fluorometric plate reader (NorthStar Scientific Ltd, Leeds, UK) with a filter setting of 485nm excitation and 530nm emission. NETs were recorded in arbitrary fluorescent units (AFU) and the mean was calculated from the 4 wells for each stimulant.
2.6. Neutrophil Apoptosis
- Neutrophil apoptosis was assessed using a commercially available assay and was carried out as per the manufacturer’s instructions. 1x106 neutrophils suspended in RPMI-1640 (Sigma-Aldrich) at a concentration of 1x106/ml were incubated for 24 hours at 37°C with 5% CO2.Following incubation the neutrophils were pelleted at 500G for 5 minutes at room temperature. The pellet was washed twice with PBS then re-suspended in Annexin Buffer (BD Biosciences) at a concentration of 1x106/ml. 1x105 neutrophils were transferred into four cytometric tubes. 5µl PE-Annexin-V, 5µl 7-AAD and 5µl PE-Annexin-V and 5µl of 7-AAD (both from BD Biosciences) were added to three of the cytometric tubes respectively. One cytometric tube remained unstained. The neutrophils were gently vortexed and incubated for 15 minutes at 25°C in the dark. 400µl of Annexin Buffer was added to each cytometric tube prior to immediate flow cytometric analysis using the Accuri-C6 (BD Accuri™).PE Annexin-V is used to determine the percentage of cells within a population that are actively undergoing apoptosis. 7-AAD is used to distinguish viable from non-viable cells. Therefore, neutrophils that stain negative for PE Annexin-V and 7-AAD are alive and not undergoing measurable apoptosis. Neutrophils that stain positive for PE Annexin-V and negative for 7-AAD are undergoing early apoptosis. Neutrophils that stain positive for both PE Annexin-V and 7-AAD are undergoing late apoptosis. Neutrophils that stain negative for PE Annexin-V and positive for 7-AAD are considered necrotic.
2.7. In-vitro Experiments with HMG-CoA Reductase Inhibitors
- Simvastatin (Sigma-Aldrich) was used in the in-vitro experiments at a concentration of 1μM which was used to represent a predicted plasma concentration following ingestion of 40mg simvastatin. The solvent Dimethyl Sulfoxide (DMSO; Sigma-Aldrich) is the vehicle control for simvastatin and neutrophils not treated with simvastatin were treated with the vehicle control. Quantification of Neutrophil Extracellular Trap Formation and Neutrophil Apoptosis assays were repeated after isolated neutrophils were incubated with 1μM simvastatin (or equivalent dilution of DMSO) for 40 minutes at 37°C with 5% CO2.
2.8. Statistical Analysis
- Statistical analysis was performed using GraphPad Prism Version 6 (La Jolla, California, USA). Continuous data was analysed using non-parametric statistical models. Mann-Whitney U tests were used for independent samples and Wilcoxon Signed Rank tests for related samples for two groups. All statistical tests performed were two-tailed and results were considered significant when p<0.05.
3. Results
- A total of 15 patients undergoing elective colorectal cancer resection, within an established enhanced recovery programme, between March 2014 and March 2015 were recruited into the study. The median age of the population was 72 years (IQR 59-80 years) and 11 patients (73.3%) were of male gender. The median BMI was 24.5 kg/m2 (IQR 21.6 – 27.8 kg/m2) and 8 patients (53.3%) had an ASA score of 3 or more. Of the recruited patients, 5 patients (33.3%) were established on statin treatment prior to enrolment into the study (all simvastatin 40mg).11 patients (73.3%) had early stage disease (Dukes’ A and B), 4 patients (26.7%) had node positive disease (Dukes’ C) and no patients had confirmed metastatic disease. 9 patients (60.0%) underwent segmental resection for colonic cancer and 6 patients (40.0%) underwent rectal resection for recto-sigmoid or rectal cancer. 10 resections (66.6%) were completed laparoscopically.10 patients (66.6%) developed a complication, although only 3 patients (20.0%) developed a significant complication (Clavien-Dindo ≥ 3). The median length of stay was 9 days (IQR: 7-16 days). 1 patient (6.7%) died within 30-days and 1 patient (6.7%) died within 90-days of the index procedure giving a 90-day mortality of 13.3%. No further deaths occurred to a median follow-up of 21.3 months (IQR: 16.7-23.5 months). The baseline population characteristics and operative characteristics are shown in Table 1.
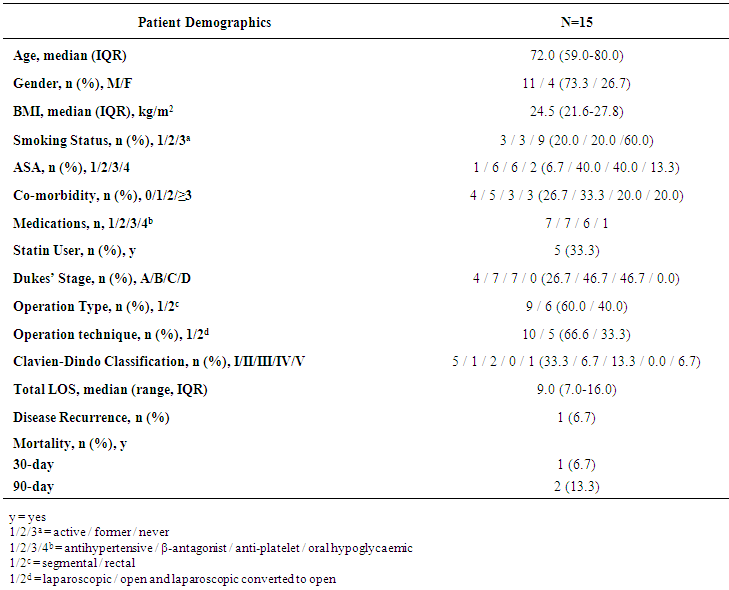 | Table 1. Population Characteristics of Patients who Underwent In-vitro Experiments with Simvastatin |
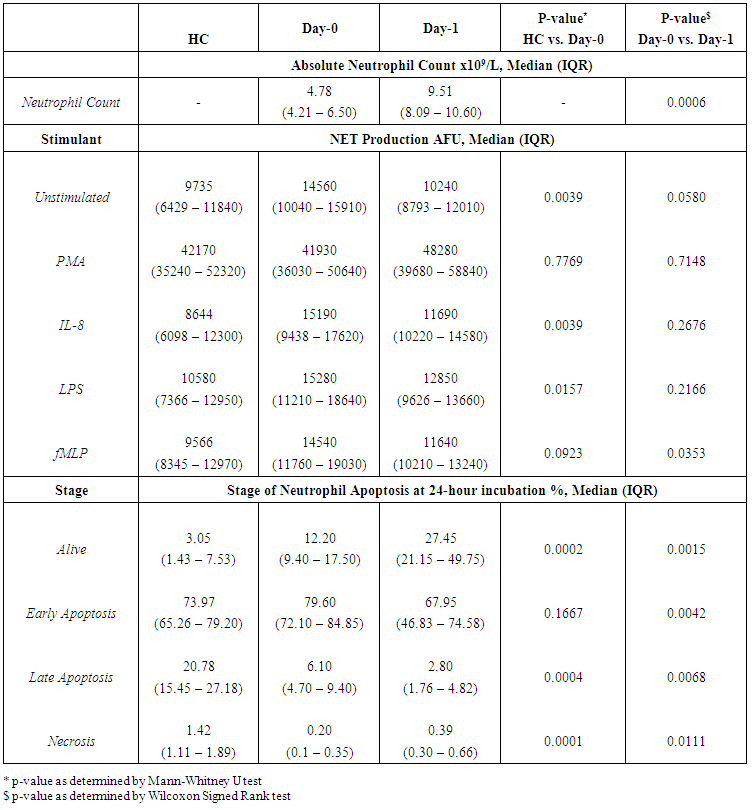 | Table 2. Absolute Neutrophil Count, Neutrophil Extracellular Trap Production and Stage of Neutrophil Apoptosis in Healthy Controls (HC) and in Patients with Colorectal Cancer on Day-0 and Day-1 |
4. Discussion
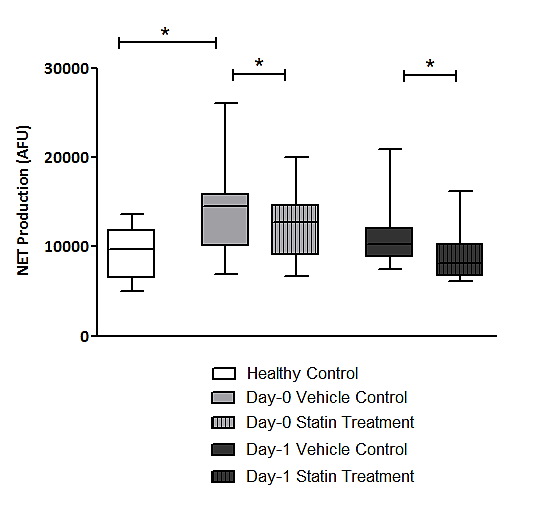
- The experiments undertaken in this study help to provide a greater understanding of the functional changes in neutrophils in patients with colorectal cancer and as a consequence of surgery. They also reveal the potential for neutrophil immune-modulation with simvastatin treatment in-vitro.Firstly, the experimental findings demonstrate that patients with a diagnosis of colorectal cancer have significantly increased NET production when compared to healthy individuals which supports the current evidence that NETs are likely to be involved in cancer development. It is known that neutrophils play a vital role in the development of metastases where neutrophils facilitate circulating tumour cell adhesion to both pulmonary and hepatic endothelial surfaces [31-34]. It is thought that the direct contact of circulating tumour cells and neutrophils is an important precursor to the development of metastatic disease [33, 34]. It is theorised that NETs may act within the primary tumour and promote tumour progression and dissemination [31]. NETs are thought to expose tumour cells to high concentrations of biologically active proteins which favour tumour proliferation and inhibit tumour cell apoptosis. It is also suggested that NETs support the dissemination of tumour cells from the primary tumour and promote early adhesive events and increase sequestration of malignant cells in end organs [33, 34]. It is also proposed that primary tumours can facilitate NET production in circulating neutrophils. This effect has been attributed, in part, to granulocyte-colony stimulating factor (G-CSF) production by tumours [36]. Furthermore, Tumour Necrosis Factor-α (TNF-α) and IL-8 have been shown to facilitate NET formation and these cytokines are highly expressed by a number of tumour types, including colorectal cancer [32, 37, 38]. A significantly impaired apoptosis was also observed in patients with colorectal cancer which supports the evidence of reduced apoptosis of neutrophils caused by their activation which is mediated by nuclear factor-kappa B which is associated with reduced activity of caspase-3 and caspase-9 [39, 40]. Secondly, a significant reduction in NET formation from Day-0 to Day-1 was detected. This observed NET reduction, as a consequence of surgery, may indicate neutrophil activation as the findings are supported by in-vitro experiments which studied the neutrophil functional changes in patients with sepsis and severe sepsis [41]. Significant changes indicating impaired apoptosis were also observed from Day-0 to Day-1 which is again indicative of an activated, ‘pro-inflammatory’ neutrophil state. It is proposed that an accumulation of activated neutrophils in the circulation could be potentially harmful to the host following surgery. In fact, during the progression of sepsis, a state of neutrophil paralysis has been observed which results in the impairment of neutrophil anti-microbial functions including chemotaxis, ROS release and formation of NETs [42]. This results in the widespread dissemination of pathogenic material and to the development of acute lung injury (ALI) and multi-organ dysfunction syndrome (MODS) [43]. The mechanisms underlying defective neutrophil function remain elusive, although NET formation has been implicated in the immunosuppression and increased rates of sepsis seen in neonates [44], whereas other studies suggest NETs may exaggerate endothelial damage [45, 46]. In a recent experimental study, in a cohort of patients undergoing attempted curative liver resection for metastatic colorectal cancer, it was observed that increased post-operative NET formation was associated with a >4-fold reduction in disease-free survival [47]. In this study, ELISA was used to quantify High Mobility Box 1 (HMGB-1) protein levels and Myeloperoxidase (MPO)-DNA complex levels from serum samples on Day-1 from patients who underwent hepatectomy for metastatic colorectal cancer. Previous studies have shown that extracellular HMGB-1, a highly conserved DNA-binding protein, plays a critical role in regulating the process of Toll-Like Receptor 9 (TLR-9) activation by circulating DNA complexes [48, 49] and it has been identified by immunofluorescence that HMGB-1 is a component of NETs [47]. MPO-DNA complexes were used to clarify that the circulating nucleosomes were derived from NETs [50, 51] and a significant strong correlation between post-operative MPO–DNA levels and HMGB-1 levels were identified. In this study it was identified that NET formation was accelerated immediately after major liver resection, by observed increases in HMGB-1 and this was associated with a significant increase in early metastatic disease. It was also observed that there was a significant increase in pre-operative HMGB-1 levels in patients with metastatic colorectal cancer compared with healthy controls [47].At first this evidence appears contrary to the evidence presented in our experimental study. In our study, however, when taking into account the number of absolute circulating neutrophils, despite an observed reduction in NET formation from Day-0 to Day-1, in controlled experiments using a pre-determined quantity of neutrophils (1x105 neutrophils), a significant increase in the ‘absolute NET production’ (a multiplication of NET production and absolute circulating neutrophils [AFU / no. neutrophils x 105/L]) was identified from Day-0 to Day-1 in response to all stimulants. This might imply that the number of circulating neutrophils are also important when determining patient outcomes and this may also explain why serum MPO–DNA and HMGB-1 levels were elevated following surgery for metastatic colorectal cancer.This finding is also supported by experimental murine studies of surgical stress [47] and sepsis [31] in the context of colorectal cancer. An increase in NET formation that correlated with an accelerated development and progression of metastatic disease was observed following the surgical stress of liver ischaemia-reperfusion [47] and a widespread deposition of NETs and the promotion of the development of gross metastases was observed in a caecal ligation and puncture model. It was also revealed that tumour cells were trapped within NETs and this was associated with the development of metastases [31].’Thirdly, neutrophil functional changes were observed when stimulated with simvastatin in-vitro. In those patients with colorectal cancer a restoration of neutrophil function (from dysregulated to normal function) was demonstrated: reduced NET production and restored apoptosis. Following surgery, a further reduction in NET production was revealed with statin treatment on Day-1. This may be advantageous to the host as the reduction in NETs following surgery theoretically reduces the tumour-neutrophil interaction and the potential for disease dissemination and progression. Indeed, the evidence which suggests a role of neutrophil immune paralysis in the development and progression of sepsis is amenable to correction with statin therapy [28-30]. As neutrophils are also appreciated to have an important role in cancer progression and dissemination the defects in neutrophil function, as a consequence of cancer or the high-grade, non-specific SIR associated with cancer surgery, may also be amenable to modulation by statin therapy. This might explain why patients taking peri-operative statins appear to have a greater baseline peri-operative risk compared to non-statin users but achieve equivalent outcomes [19, 22] and why there is a reduced risk of a variety of cancer types [52-54] and a reduced all cause and cancer specific mortality [55] associated with statin use. This theory is supported further by a recently published prospective, double-blind, placebo-controlled RCT, from Aukland, New Zealand, which investigated the peri-operative use of simvastatin in the context of major colorectal surgery, including both malignant and benign resectional surgery [56]. Although numbers of study participants were relatively small (65 simvastatin vs. 67 placebo) it was identified that peri-operative statin therapy attenuates the early pro-inflammatory response to surgery with significant reductions in plasma concentrations of IL-6, IL-8 and TNF-α and peritoneal concentrations in Il-6 and IL-8 on Day-1. Despite a reduction in plasma and peritoneal pro-inflammatory cytokines no differences in post-operative complications were identified (OR 0.71, 95% CI 0.33-1.52, p = 0.444) [58]. This study provides evidence to explain the neutrophil functional changes identified in our experimental study and the potential for immune modulation by simvastatin. IL-6, IL-8 and TNF-α are all implicated in the acute phase reaction of systemic inflammation and are responsible for the production of neutrophils, neutrophil migration and degranulation, and are produced by activated neutrophils. These pro-inflammatory cytokines help to explain the neutrophil phenotypes, which exhibit; increased NET formation and reduced apoptosis in patients with colorectal cancer when compared to a healthy control population, reduced NET formation and reduced apoptosis in response to surgery, and attenuated function, from dysregulated to normal function, with in-vitro treatment with simvastatin.Our experimental study has a number of limitations. Firstly, the population evaluated was small and heterogeneous with regard to patient demographics, patient co-morbidities, tumour characteristics and operative characteristics making interpretation of the results difficult and limiting the generalisability of the study findings. Secondly, in-vitro experiments performed to assess neutrophil function were conducted outside of the biological context and consequently there are challenges in extrapolating the results and it must be acknowledged that they cannot be readily transposed to, and predict the reaction of, the entire organism in-vivo. Thirdly, patients on established statin therapy were not excluded from the study. 5 patients (33.3%) were taking statins (all simvastatin 40mg) at the time of surgery and could therefore potentially be in established ‘pleiotropy’. The in-vivo effect of statin therapy on neutrophil function is therefore not quantified in the in-vitro experimentation on neutrophil function. Importantly, no significant differences were identified on Day-0 and Day-1 between those patients on established statin therapy and those patients not on established statin therapy before or after treatment with 1μM simvastatin in both Quantification of Neutrophil Extracellular Trap Formation and Neutrophil Apoptosis assays. Lastly, neutrophils were treated with simvastatin in experimental conditions in-vitro and this may not replicate the effect on systemic neutrophils in-vivo, also the 1μM concentration of simvastatin used was a predicted concentration corresponding to a plasma concentration following ingestion of 40mg simvastatin. The benefit of in-vitro experimental methods in this context is that each patient acted as its own control and the true impact of simvastatin treatment was detected. This was supported further by the use of the vehicle control for simvastatin in the non-treatment group which assisted in determining the true impact of simvastatin on neutrophil function.
5. Conclusions
- As surgical resection remains the mainstay of treatment for the majority of solid tumours it is appreciated that an uncontrolled inflammatory response should be avoided in an attempt to minimise damage to the host. The evidence suggests that it is conceivable to implement a therapeutic strategy in the peri-operative period to reduce the systemic inflammation associated with surgery and improve oncological outcomes. The interaction between tumour immunity and systemic immunity is likely to be complex, but the administration of immune-modulatory therapies in an attempt to modify the peri-operative inflammatory response could be utilised to enhance the anti-tumour immune competency of the host and merits further investigation in the context of cancer surgery. A therapeutic strategy aimed at modifying tumour-neutrophil interactions may maximise the therapeutic benefit of surgery. Statins are promising immune-modulatory agents in the context of cancer surgery.
ACKNOWLEDGEMENTS
- With grateful thanks to the staff of the General Surgical Department, Heart of England NHS Foundation Trust, Birmingham, UK and to the staff of the Institute of Inflammation and Ageing, College of Medical and Dental Sciences, The University of Birmingham, UK.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML