-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Surgical Research
p-ISSN: 2332-8312 e-ISSN: 2332-8320
2016; 5(2): 21-25
doi:10.5923/j.surgery.20160502.03

Audit of Perineal Wound Healing after Primary Closure of the Perineal Wound without Perineal Drainage in Abdominal Perineal Resection for Malignancy
Elroy P. Weledji 1, J. Palmer Frcs 2
1Department of Surgery, Faculty of Health Sciences, University of Buea, Cameroon
2Department of Surgery, Cumberland Infirmary, Carlisle, UK
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

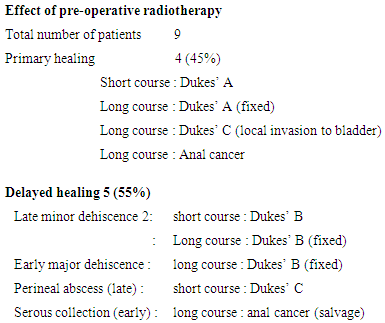
This audit has retrospectively assessed the result of primary closure of the perineum irrespective of risk factors for healing: pre-operative radiotherapy, faecal contamination, age, advanced malignancy and diabetes mellitus. 74 consecutive patients (49 males and 25 females median age 70 years) with rectal carcinoma (72) and anal carcinoma (2) who had undergone abdominal perineal excision from August 1989 to December 1999 were identified retrospectively. Complications of the perineal wound were tabulated. Fifty five of 74 patients (75%) healed primarily by two weeks and delayed healing occurred in 19 (25%) patients including 3 (4%) with complete dehiscence. Four of nine patients (45%) who received pre-operative radiotherapy achieved primary healing. Two of 32 patients (6%) who received post-operative radiotherapy after the perineal wound had healed developed wound complications. The primary healing rate of the advanced tumours Dukes’ stage B &C (74%) was similar to the overall healing rate. The average length of hospital f stay following primary healing was 12.25 days and 17.05 days following delayed healing. Other factors comparing tumour stage, sex, age and weight were not statistically significant. A technique of complete primary closure (“sealing”) of the perineal wound by obliterating the perineal dead space over a transabdominal suction drain can provide a primary healing rate of 75% by two weeks irrespective of risk factors. Primary closure is safe and can be advised in most cases.
Keywords: Anorectal cancer, Abdominal perineal resection, Primary closure (“sealing’’ of the perineum), Trans-Abdominal suction drain, Primary wound healing, Risk factors
Cite this paper: Elroy P. Weledji , J. Palmer Frcs , Audit of Perineal Wound Healing after Primary Closure of the Perineal Wound without Perineal Drainage in Abdominal Perineal Resection for Malignancy, International Journal of Surgical Research, Vol. 5 No. 2, 2016, pp. 21-25. doi: 10.5923/j.surgery.20160502.03.
Article Outline
1. Introduction
- There is overwhelming evidence to support the superiority of perineal wound closure with suction drainage compared with leaving wounds open following abdominal perineal excision for malignancy provided there is no pre-existing sepsis and there has been no feacal contamination during rectal excision [1-10]. Morbidity is substantially reduced by leaving the wound open to granulate in patients with known risk which are associated with wound breakdown. These factors include previous radiotherapy, age advanced malignancy, diabetes mellitus and inadequate haemostasis [1-3]. In this retrospective series, all wounds were closed initially except in one patient with pre-existing perineal sepsis and two patients with uncontrollable haemorrhage in whom delayed primary closure was done. By complete obliteration of the perineal dead space over a trans-abdominal suction drain which reaches into the perineum and opposing the skin edges subcuticularly we propose that a clean, dry and “sealed” perineum without an exiting perineal drain reduces infection and it is probably more comfortable for the patient. This study reported the result of primary closure of the perineum irrespective of risk factors following abdominal perineal excision for malignancy within a ten year period at the Cumberland Infirmary, Carlisle, UK.
2. Patients and Methods
- In the ten year period between August 1989 and December 1999, 74 consecutive patients underwent abdomino-perineal resection for malignancy (72 for rectal carcinoma and two for anal carcinoma) at the Cumberland Infirmary, Carlisle. Some of the patients with operable tumours had received a short course of pre-operative adjuvant radiotherapy (5Gy for 5 days) while those with fixed rectal tumours had received a long course of radiotherapy. Those with involved resection margins had post-operative radiotherapy after the perineal wound had healed. The patients were given no bowel preparations before the operation. Prophylactic antibiotics in the form of a second generation cephalosporin and metronidazole were commenced pre-operatively and continued for three days. Prophylaxis against deep vein thrombosis was carried out in the form of subcutaneous heparin 5000 iu bd, TED stockings and the application of a pneumatic calf compression device in theatre. A standard operative technique was carried out. With the patient in the Lloyd-Davies position, the tumour operability was assessed by the abdominal operator who then commenced rectal mobilization from above. The perineal excision was elliptical, extending posteriorly to the tip of the coccyx. In the female patient the incision incorporated the posterior vaginal wall anteriorly, in the male patient it terminated anteriorly at the level of the perineal body. Mobilization of the rectum was completed from below with the rectum being delivered through the perineal wound. Meticulous perineal haemostasis was ensured. Measures were taken to avoid faecal contamination, including suturing of the anal orifice and staple transection of the rectum.The pelvic peritoneum was closed from above only when possible with a continuous absorbable suture over a suction drain (redivac) inserted trans-abdominally to reach the perineum. Below, the remnant perineal muscles, ischiorectal fat and subcutaneous tissue were approximated with interrupted vicryl and the perianal skin was carefully approximated using continues subcuticular vicryl suture. The wound was sprayed with opsite and dressed. There was no variation in the surgical method as it was carried out or supervised by the same surgeon. The perineal wound was inspected daily by the medical staff for signs of cellulitis, collection, (haematoma, seroma, abscess) and dehiscence. If there was evidence of a persistent cavity or haematoma which was not draining adequately in the presence of a partially healed skin wound, the primary closure was converted into an open wound by removal of all superficial and deep sutures to allow healing by secondary intention. The suction was removed when it ceased to drain significant amounts of blood or serum which was usually from the fifth post-operative day. In the event of incomplete healing at the time of discharge, the progress of the wound was assessed at the follow- up clinic.
3. Method
- In a retrospective examination of individual patients’ case notes, the following data was recorded: age, sex, stage of tumour, pre or post-operative radio/chemotherapy, pre-operative risk factors (faecal contamination, locally advanced tumour, haematoma), co-morbidity, (body weight, diabetes mellitus, COAD and cardiac risk factors) post-operative complications were recorded as general or specific to the perineal wound.Complications of the perineal wound were tabulated as, infection which included cellulitis or discharge of purulent material, collection (haematoma, seroma and abscess) and dehiscence which is recorded as minor when no deeper than skin level and involving less than 10% of the entire length of the wound and major when between 10% and 100% of the length of the perineal wound dehisced, with the dehiscence being confined to the skin and immediate sub-dermal tissue. Complete dehiscence was recorded when the entire wound broke down.Primary healing was recorded when the entire length of the perineal wound was healed within two weeks. The wound was considered to be healed once dressings were no longer required. The occurrence of wound dehiscence as early (< 2 weeks) or later (>2weeks) were noted. The length of hospital stay, the state of the wound at first follow-up and late complications- delayed wound healing, perineal sinus and perineal hernia were recorded.
4. Results
- Of the 74 patients, 49 were male and 25 were female with an age range of 50-85 years (median 70 years)1. Healing of the Perineal Wound (table 1)Primary healing of the perineal wound within two weeks occurred in 55 (75%) patients (P<0.05). Delayed healing occurred in 19 patients (25%). This consisted of 2 serous collections, 2 perineal haematomas, 1 pelvic haematoma, 1 perineal abscess, 10 perineal dehiscence and 3 perineal sinuses.
|
|
5. Discussion
- Historically, perineal wounds have been managed without primary closure and healing allowed to occur by secondary intention. This will eventually yield good results but it is inconvenient to the patient and time consuming.Many authors have suggested that primary closure is safe in the absence of risk factors for healing and have applied individual modifications that they feel would yield better results (figure 1) [4-17]. The incidence of primary healing of the perineal wound following abdominal perineal excision for malignancy ranges from 37% to 92% reflecting the widely different patient selection [4, 17]. For example Altemier et al 92% healing rate was confined to carefully selected patients who did not have extensive tumour, pre-operative radiotherapy or intra-operative rectal damage, whereas Baudot et al 41% healing rate was for all patients [7, 15].
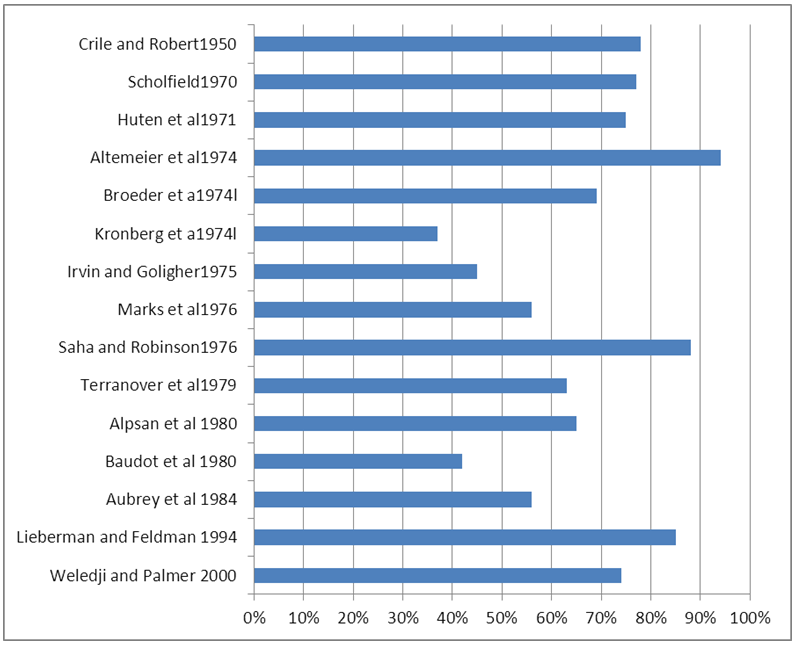 | Figure 1. Incidence of primary perineal wound healing after abdominoperineal excision of the rectum for cancer with primary closure over suction drains |
6. Conclusions
- Our retrospective study of 74 patients who had undergone abdominal-perineal resection for malignancy have demonstrated a technique of complete primary closure of the perineal wound by obliterating the perineal dead space over a trans-abdominal suction drain and subcuticularly suturing the perinal skin can provide a primary healing rate of 75% by two weeks irrespective of risk factors.This method of “sealing” the perineum without an exiting perineal drain is safe, efficacious and also provides the benefits of increased patient comfort, clean and dry wound healing and immediate ambulation. Primary closure of the perineal wound can be advised in most cases.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML