-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Surgical Research
2013; 2(3): 16-20
doi:10.5923/j.surgery.20130203.02
Management of Bleeding Duodenal GIST
Michael Arvind, Narasimman Sathiamurthy, Tan Wee Jin
Department of Surgery, Hospital Pulau Pinang, Jln Residensi, 10990, Penang, Malaysia
Correspondence to: Narasimman Sathiamurthy, Department of Surgery, Hospital Pulau Pinang, Jln Residensi, 10990, Penang, Malaysia.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
In 1983, Mazul and Clark introduced Gastrointestinal Stromal Tumors (GIST). The term encompasses mesenchyme tumors of the GI tract, mesentery and retroperitoneum. Its incidence is estimated to be 10-20 per million people and occur mostly in adults aged above 40 years with a median age of 63 years. GISTs are most often seen in the stomach (50-70%), small intestine (25-30%), colon and rectum (5-10%), and esophagus (<5%). Duodenal GIST is rare (3-5%). GIST has an overall malignant potential in 20-30% of cases. Patients with duodenal GIST usually present with abdominal pain, nausea and vomiting. Occasionally they can present with upper GI bleed. This paper describes two differing cases of bleeding duodenal GIST. One presenting with life threatening hemorrhage and another as chronic gastrointestinal hemorrhage.
Keywords: Dudoenal, GIST, Bleeding
Cite this paper: Michael Arvind, Narasimman Sathiamurthy, Tan Wee Jin, Management of Bleeding Duodenal GIST, International Journal of Surgical Research, Vol. 2 No. 3, 2013, pp. 16-20. doi: 10.5923/j.surgery.20130203.02.
Article Outline
1. Introduction
- In 1983, Mazul and Clark introduced Gastrointestinal Stromal Tumors (GIST). The term encompasses mesenchyme tumors of the GI tract, mesentery and retroperitoneum[5]. Its incidence is estimated to be 10-20 per million people and occur mostly in adults aged above 40 years with a median age of 63 years. GISTs are most often seen in the stomach (50-70%), small intestine (25-30%), colon and rectum (5-10%), and esophagus (<5%)[2]. Duodenal GIST is rare (3-5%)[10].GIST have on overall malignant potential in 20-30% of cases[1].Patients with duodenal GIST usually present with abdominal pain, nausea and vomiting[4].Occasionally they can present with upper GI bleed[5].This paper describes two differingcases of bleeding duodenal GIST. One presenting with life threatening hemorrhage and another as chronic gastrointestinal hemorrhage.
2. Case Report
2.1. Case Report 1
- Mr. N, a 65 year old Chinese gentleman with underlying diabetes, hypertension and dyslipidemia presented with complaints of passing malenic stools intermittently, every 2-3 days over the preceding 3 months. Hepresented with symptoms of lethargy, dizziness and 2 episodes of coffee ground vomitus. He was anemic with a hemoglobin count of 7.4g/dL.Further history showed that he was on ticlopidine for 15 years but had stopped 2 weeks prior to presentation.Anoesophagogastroduodenoscopy (OGDS)showed only mild gastritis changes involving the antrum of the stomach.Colonoscopy was unremarkable. Anabdominal ultrasound showed a suspicious bowel mass in the sub hepatic space and a contrast enhanced CT scan of the abdomen showed a lobulated cavitating mass with pockets of air within the right hypochondria with splaying of the adjacent duodenum.The mass was not distinguishable from the adjacent duodenum (Figure 1).The differential diagnosis at this juncture wasduodenal GIST, duodenal diverticulum or lymphoma.Surgical exploration led to a Whipple’s procedure and en bloc right hemicolectomy with excision of a liver nodule.Intraoperative findings showed a tumor measuring 5 x 7 cm adhered to the duodenum and proximal transverse colon with a 3 x 2 cm nodule in segment II of the liver. The tumor had focal erosion into the duodenum just distal to the ampulla of Vater, which was the cause of the bleeding (Figure 2). There was no lymphadenopathy.Histopathological report of the en-bloc resection of tumor with duodenum, pancreas, jejunum and attached ileum and colon was confirmed as high grade GIST with expression of CD117 and mitotic figures seen in 10/10 high powered field. The resection margins were clear of tumor cells. The liver nodule also had GIST cells.Postoperative recovery was uneventful. He is currently on oral Imatinib.
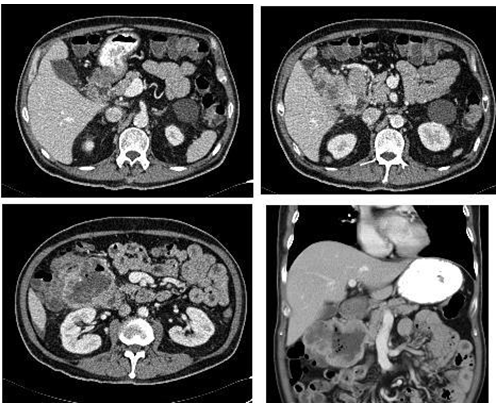 | Figure 1. Axial and coronal view of intravenous and oral contrasted CT scan of the abdomen showing the duodenal mass |
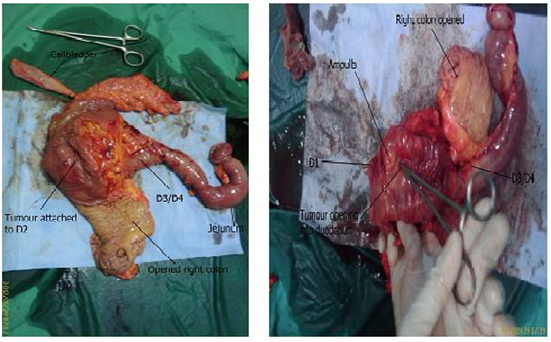 | Figure 2. Resected specimen of the pancreaticoduodenectomy |
2.2. Case Report 2
- Mrs. C, a 56 year old lady with no underlying medical illness presented with a two day history of multiple episodes of coffee ground vomitus and passing blackish stool for a day. She also complained of upper abdominal pain and symptoms of anemia. There was no other significant history. Clinically she was pink, her vitals were stable and her abdominal findings were equivocal. Per rectal examination showed melena.
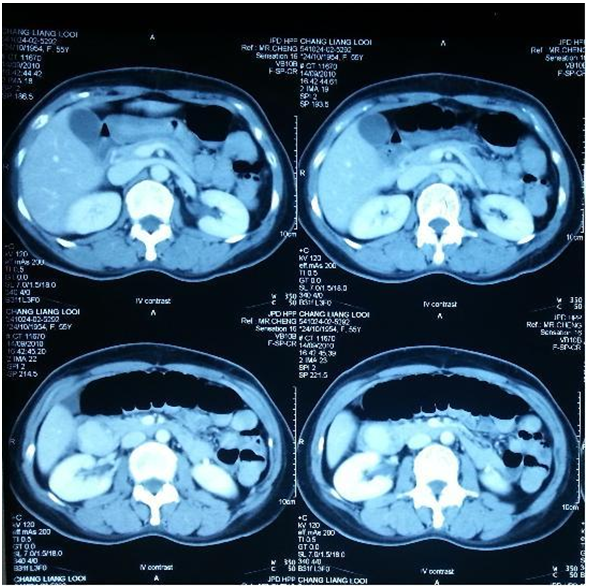 | Figure 3. Axial views of the abdominal CT showing hypodense lesion between D1/D2 |
3. Discussion
- GISTs aremesenchyme tumors of gastrointestinal tract and are believed to originate from the neoplastic transformation of the interstitial cells of Cajal from their precursors[4].GISTs develop due to uninterrupted action of the tyrosine kinase enzyme. They have strong CD117 expression, often express CD34 and sometimes express alpha-smooth muscle actin (SMA)[4].Duodenal GIST is rare (3-5%), but the commonest presentation is bleeding (75%), as compared to stomach GIST (54%)[5]. The second part of the duodenum is most commonly involved (49%),as seen in these cases, followed by the third, fourth and first part[5].In the first patient, although the upper GI endoscopy was normal, inspection of the resected specimen showed a small opening into the duodenum just distal to the ampulla, which could have been easily missed due to position of the opening while viewing it endoscopically. The second patient showed a mass that was initially thought to be an ulcerated polyp at the D1/D2 junction. In most cases, upper endoscopy could easily detect the lesion if there is an endoluminal extension[5].In CT scans, GIST usually is well defined heterogeneous mass with peripheral enhancing border of variable thickness and central low attenuation. This was shown in both patients around the D2 region anterior to the pancreatic head[6]. With these findings along with the symptoms of an upper GI bleed, the decision was made for a pancreaticoduodenectomy in both patients.Endoscopic ultrasound (EUS) is another mode of investigation that can be used to diagnose the duodenal mass and biopsy maybe taken[4]. It will show a homogenous hypoechoic mass in bowel wall.[6]. Achieving a diagnosis solely on EUS is difficult, however when combined with fine needle aspiration cytology (FNAC) it is useful in attaining a diagnosis but still does not provide sufficient data regarding malignant potential of the tissue.[7]. M.S Kwon et al. performed FNAC using ultrasound guidance or CT scan and the histological sections were grouped according to the criteria of Lewin et al. He managed to demonstrate that FNAC cytology;cellblock and immunohistochemistry can confidently provide a diagnosis of primary GIST[2]. This has led to introduction of tru-cut biopsy to provide complete diagnosis; however there is only limited data to support its use[7]. In some cases, the duodenal mass can be masquerading as a pancreatic head tumor and EUS will be able to differentiate them[5]. Current data still shows EUS with FNAC or tru-cut biopsy does not significantly alter the management of GIST tumors with surgery still the mainstay of treatment for large GIST[7]. In the first case, it was not indicated, as the CT scan was adequate to reach a diagnosis and a surgical decision. The second patient was actively bleeding which precluded the decision for surgical intervention.Transarterial embolization can be attempted in the initial management of a bleeding duodenal GIST[4, 9]. In our first patient, he was having occasional small amounts of bleeding that caused him to have malena. In the second patient, she presented with severe acute bleeding. Embolization was not attempted as she was clinically unstable and required urgent surgical intervention. With the presence of an operable mass seen in both the CT scans, extirpative surgery was done with an intention to cure.The choices of surgery previously done for duodenal GIST are local resection or pancreaticoduodenectomy[3]. The goal of the surgery is complete en bloc resection of the tumor with avoidance of tumor rupture to prevent peritoneal seedlings[3]. Local resection can be done in small tumors away from the ampulla with a duodenal lumen size adequate for primary closure[5]. Segmental resection can be done with duodenojejunostomy reconstruction[5]. If near the ampulla, or the mass is occupying the head of pancreas, then a pancreaticoduodenectomy is the surgery of choice[5]. In our first case, tumor was attached to the head of pancreas and eroding just distal to the ampulla, there was also a single liver nodule in segment II. Pancreaticoduodenectomy and local resection of the liver nodule was performed. The second case had the tumor just next to the ampulla and after a wide excision of the tumor the minor ampulla was not visualized hence the decision for a Whipple’s resection. Metastectomy is shown to improve survival in GIST tumor. The 5-year survival rate was 35-65% following complete resection and the medial survival rate was 10-20 months for unresectable disease[8].Traditionally, GIST has poor response rate to chemotherapy, less than 10%[8]. The emergence of Imatinibmesylate is an oral tyrosine kinase inhibitor that inhibits the KIT and PDGFR tyrosine kinases as well as other members of the type III group of tyrosine kinases[1]. This has revolutionized the treatment of GIST and renders longer progression free survival, especially in metastatic, unresectable or recurrent GISTs[1]. However, after about 18 to 24 months on treatment, they may develop resistance[8]. After 5 years of treatment with Imatinib, most patients will have recurrence[8]. At this point, another oral tyrosine kinase inhibitor, sunitinib, can be used as an alternative[8]. With the availability of Imatinib and Sunitinib, patients diagnosed with metastatic GIST can be treated preoperatively before embarking on a major resection[10]. Before this, surgery was the mainstay of treatment, with overall survival of 10 to 20 months and 5-year survival of less than 10%[8]. With preoperative Imatinib, the median survival has been reported to be reaching 5 years[1]. In our first patient, there was no finding suggestive of a metastatic disease preoperatively, thus no Imatinib given. He was only started on Imatinib after noting a high-grade tumor and the liver nodule resected was proven to be metastatic GIST. Low grade GIST tumors are not indicated to be treated with Imatinib.
4. Conclusions
- These two cases are of bleeding duodenal GIST. Both the cases presented opposite ends of a spectrum, one with active bleeding duodenal mass and the others with no endoluminal mass with chronic bleed. Duodenal GIST is rare and can be missed during endoscopy. CT scan of the abdomen is necessary to make the diagnosis. In cases of suspicion, EUS can be used. Surgery is the mainstay of treatment in cases of acute bleeding and where metastasis is not evident. Imatinib can be administered preoperatively if metastatic GIST is diagnosed. Postoperative Imatinib renders improved progression free survival and overall survival.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML