-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Surgical Research
2013; 2(1): 1-3
doi:10.5923/j.surgery.20130201.01
Case Report: Disseminated Intravascular Coagulation (DIVC) Secondary to Surgical Management of Gastric Adenocarcinoma
Andee Dzulkarnaen Zakaria1, Amer Hayat Khan2, Syed Hassan1, Mak Woh Yon2, Khurshid Alam Wazir2
1Department of Surgery, School of Medical Sciences, Universiti Sains Malaysia, Health Campus, 16150, Kelantan, Malaysia
2Department of Clinical Pharmacy, School of Pharmaceutical Sciences Universiti Sains Malaysia, 11800, Penang, Malaysia
Correspondence to: Andee Dzulkarnaen Zakaria, Department of Surgery, School of Medical Sciences, Universiti Sains Malaysia, Health Campus, 16150, Kelantan, Malaysia.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Gastric adenocarcinoma is a cancer which emerges from the gastric tissue. More than 90% of gastric cancers are adenocarcinomas. Disseminated intravascular coagulation (DIVC) is characterized by a widespread activation of coagulation, resulting in increased intravascular formation of fibrin and thrombotic occlusion of small and mid-size vessels. A 47 years old Malay man, diagnosed with gastric adenocarcinoma stage III was admitted to the surgical ward for preparation of curative total gastrectomy operation. Intraoperative findings noted advanced gastric adenocarcinoma with distant metastasis. Patient underwent laporatomy, feeding jejunostomy and cholecystojejunostomy as palliative management. However, this condition was further complicated by acute disseminated intravascular coagulation (DIVC) which required the DIVC regimen of 4 units of fresh frozen plasma, 6 units cryoprecipitate and 2 units of platelet concentrate. Surgical resection is the only curative treatment for gastric cancer. Gastric cancer diagnosed at an advanced stage has poor prognosis, hence early diagnosis is crucial in influencing the outcome of surgical treatment. DIVC is known as secondary complication of an underlying disease among cancer patients, and precautions are imperative elements. Treatment of DIVC may include platelets, red blood cells, plasma cryoprecipitate transfusions, and antibiotics.
Keywords: Gastric Adenocarcinoma, Disseminated Intravascular Coagulation, Radiotherapy, Surgery, Chemotherapy
Cite this paper: Andee Dzulkarnaen Zakaria, Amer Hayat Khan, Syed Hassan, Mak Woh Yon, Khurshid Alam Wazir, Case Report: Disseminated Intravascular Coagulation (DIVC) Secondary to Surgical Management of Gastric Adenocarcinoma, International Journal of Surgical Research, Vol. 2 No. 1, 2013, pp. 1-3. doi: 10.5923/j.surgery.20130201.01.
1. Introduction
- Gastric adenocarcinoma is a cancer which emerges from the gastric tissue. More than 90% of gastric cancers are adenocarcinomas.[1] Gastric cancer is the fourth most common cancer and the second most common cause of cancer deaths worldwide, accounting for an estimated 620, 000 deaths per year.[2,3] It is the number seven most common cancer among males in Malaysia.[2] Risk factors for gastric cancer include Helicobacter pylori infection, male sex, a family history of gastric cancer and smoking.[2] Treatment options include radiotherapy, surgery and chemotherapy. Disseminated intravascular coagulation (DIVC) is often observed in advanced cancer patients due to acute development of disseminated fibrin microthrombi and may result in hemostatic abnormalities manifested as haemorrhage or thrombosis. The incidence of DIVC in consecutive patients with solid tumours was found to be 7% in a recent clinical study.[4] DIVC develops more slowly in cancer patients and clots in veins are more common than bleeding, as seen in current patient. Intravascular coagulation can compromise the blood supply to organs and may cause multiple organs failure, while subsequent depletion of platelets and coagulation proteins from the ongoing coagulation may induce severe bleeding.[5] DIVC is characterized by widespread activation of coagulation, resulting in increased intravascular formation of fibrin and thrombotic occlusion of small and midsize vessels.[6]
2. Case Presentation
- A 47 years old Malay man, diagnosed with gastric adenocarcinoma stage III was admitted to the surgical ward in Hospital Universiti Sains Malaysia, for gastrectomy. For last two months, the patient complained of abdominal pain likes obstructing stomach. Patient lost eight kilogram body weight in 2 months time due to loss of appetite and food intolerance. Patient had maelenic stools on and off. Patient has positive family history of cancer; father suffered from esophageal cancer while mother had ovarian cancer. Patient has no known drug or food allergy, but has been chronic smoker from the past 25 years and active alcoholic user for the last 3 years. Abdominal ultrasound (Figure 1) and computedtomography (CT) scan (Figure 2) were performed to confirm the diagnosis. Abdomen ultrasound showed dilated biliary trees mass compression while CT scan showed heterogeneous enhancing nodular thickening of stomach wall. Patient condition was further complicated by acute disseminated intravascular coagulation which was diagnosed based on laboratory data.
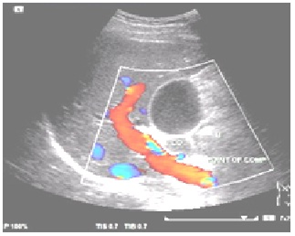 | Figure 1. Abdomen ultrasound |
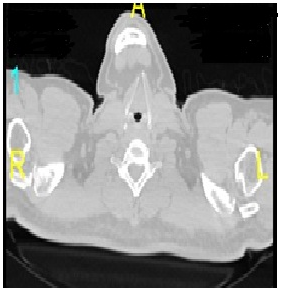 | Figure 2. CT scan of Neck |
3. Treatment
- Patient underwent laporatomy, feeding jejunostomy and cholecystojejunostomy as palliative management. Initial management before surgery included restriction of oral intake and IV fluids infusion containing NaCl 0.9% and Dextrose 5%. Patient has also completed one cycle of DIVC regimen which includes 4 units of fresh frozen plasma, 6 units cryoprecipitate and 2 units of platelet concentrate. After the surgery, patient was given IV meperidine (Narcotic analgesic) immediately, for post-operative pain and was started on epidural cocktail of analgesics continuous infusion at 8 mL/hr for 2 days until the pain eventually subsided. Patient was also started on IV metronidazole 500 mg TDS and IV cefoperazone 1 g BD. Patient was given IV pantoprazole 40 mg BD for stress-ulcer prophylaxis and IV parecoxib 40 mg BD for analgesia. Patient was given alprazolam 0.25 mg which increased to 0.5 mg the day after to induce sleep as patient is unable to sleep. Subcutaneous fondaparinux 2.5 mg ON and graduation compression stocking has been prescribed to the patient as he is at risk of developing venous thrombo-embolism due to cancer, bed-ridden and disseminated intravascular coagulation.
4. Discussion
- Upon admission, the patient was alert and conscious with mild sclera jaundice. His alanine transaminase (ALT) was 207U/L, more than 3 times the upper normal limit of ALT (0-35U/L) and alkaline phosphatase (ALP) was 792U/L which implied obstructive jaundice (normal range 40-150U/L). Total bilirubin was 72μmol/L and conjugated bilirubin was 58μmol/L, which indicated obstructive jaundice as well. These indicated that the tumour was probably blocking the bile duct, and after the cholecystojejunostomy operation, the laboratory data showed improvement. The patient’s haemoglobin was low, 10.5g/dL on admission and continued to drop even after the operation. Patient was transfused with 500 mL of packed red blood cells to normalize his haemoglobin level. His hematocrit was 34.4 and mean cell hemoglobin was 24.4, both lower than normal range. His platelet count was high initially, 433U/L (normal range 150-400U/L) but showed a continuous drop. His prothrombin time was 14.1s, longer than the usual time of 10-13.5s.[7] This may indicate unfunctional platelets as seen in disseminated intravascular coagulation (DIVC). He was started on the DIVC regimen of 4 units of fresh frozen plasma, 6 units cryoprecipitate and 2 units of platelet concentrate before the operation. The main therapy to treat DIVC is to treat the underlying condition, which is gastric cancer.[4] After the operation, epidural cocktail of bupivacaine 0.1% and fentanyl 12mg was given as patient complained of severe pain during coughing (pain score 6/10) and mild pain while at rest (pain score 1/10). One day after the operation, his pain score was 5/10 and thus IV parecoxib 40mg bd was given for two days. Parecoxib is a parenteral COX-2 selective inhibitor which is effective in treating postoperative abdominal pain. It has no significant effects on platelet function or the upper gastrointestinal mucosa.[8]Adequate prophylaxis is also needed to eliminate the risk of venous thromboembolism,[6] thus subcutaneous fondaparinux was given 12 hours after patient’s epidural catheter was removed. Fondaparinux is a low molecular weight heparin which has less bleeding tendency compared to unfractionated heparin.[9] Postoperative antibiotics are required to prevent wound infection. Patient was given antibiotics IV cefoperazone 1g twice daily and IV metronidazole 500mg three times daily. Cefoperazone is a third generation cephalosporin which has wide coverage against gram negative aerobic and anaerobic bacteria. Metronidazole is only active against anaerobic organisms and is usually used in combination for treatment of anaerobic bowel flora. Treatment of DIVC may include platelets, red blood cells, plasma cryoprecipitate transfusions, and antibiotics.[10] Therefore, proper preoperative preparation, faultless surgical skills and postoperative care are essential to reduce complications and the need for reoperation.[11] So all these precautionary elements are important to adopted and to avoid such condition.
5. Conclusions
- Gastric cancer diagnosed at an advanced stage is highly challenging. Surgical resection is the only curative treatment for gastric cancer, whereas early diagnosis is crucial in influencing the outcome of surgical treatment. Disseminated intravascular coagulation (DIVC) is one of the secondary complications of cancer. Treatment in such condition may include platelets, red blood cells, plasma cryoprecipitate transfusions, and antibiotics.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML