-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Stroke Research
2014; 2(1): 6-14
doi:10.5923/j.stroke.20140201.02
Prognostic Significance of Serum Uric Acid and Mortality Outcomes in Patients with Acute Cerebrovascular Ischemic Stroke
Charan Reddy KV, Jaishree Ghanekar
Department of Internal Medicine, MGM Medical College and Hospital, Kamothe, Navi Mumbai, India
Correspondence to: Jaishree Ghanekar, Department of Internal Medicine, MGM Medical College and Hospital, Kamothe, Navi Mumbai, India.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Cerebrovascular ischemic stroke (CVIS) is the leading cause of death worldwide after cardiovascular disease (CVD) and cancer. Raised serum uric acid (SUA) is the major risk factor for CVIS. However, the relationship between SUA and CVIS is not clear, whether the association is casual or circumstantial. In this case control study, we sought to investigate the prognostic role of SUA in acute CVIS patients and its association with stroke outcomes. Twenty five CVIS patients (16 males & 9 females) and same number of age matched control subjects were recruited for this study. CVIS male patients showed significantly (P<0.001) higher SUA levels than CVIS female patients. A negative correlation was observed between age and SUA levels. We also found a significant inverse correlation of SUA levels with HDL-c, and a positive correlation with triglycerides (r = -0.247; P = 0.001 for HDL-c and r = 0.286, P = 0.001 for triglycerides). Mean SUA levels in CVIS patients were higher on admission, and its association with mortality remained after the adjustment for covariates (Hazard Ratio (HR) = 1.21, 95% confidence interval (CI) 1.07-1.45; P = 0.001). In some of the patients (5 males & 2 females) whose SUA levels were high on admission, consistently raised further during ICU stay were died. These patients belonged to National Institute of Health Stroke (NIHS) Score -9 or quartile 3/4 as per Kaplan–Meier analysis. In conclusion, present results suggest that SUA levels were correlated with all-cause death in CVIS patients. The strong association of SUA levels with CVIS males, compared with the much lesser degree in females regardless of metabolic risk variables, highlights the necessity of intervention studies to determine whether SUA is a potential therapeutic target or mear a prognostic marker of mortality risk.
Keywords: Serum uric acid, Acute cerebrovascular ischemic stroke, Mortality, Survival
Cite this paper: Charan Reddy KV, Jaishree Ghanekar, Prognostic Significance of Serum Uric Acid and Mortality Outcomes in Patients with Acute Cerebrovascular Ischemic Stroke, International Journal of Stroke Research, Vol. 2 No. 1, 2014, pp. 6-14. doi: 10.5923/j.stroke.20140201.02.
Article Outline
1. Introduction
- Ceribrovascular ischemic stroke (CVIS) is one of the leading causes of mortality worldwide [1]. It has been reported that, globally every year 16 million people suffer a stroke, more than 5.7 million die and 5 million are permanently disabled [2, 3]. Stroke can occur to anyone at any time, regardless of age, sex or race. According to the American Stroke Association’s website, “A stroke occurs when arteries which carries oxygen to the brain cells is either narrowed or clogged by a clot (ischemic stroke) or bursts (hemorrhagic stroke). About one-third of all strokes are preceded by transient ischemic attacks (TIAs), or mini-strokes, which temporarily interrupt blood flow to the brain [4]. Stroke is the main cause of disability and mortality among the aging population, and about 87% of all cases are ischemic stroke while 15% are hemorrhagic stroke [5, 6]. In India, stroke prevalence is 90-222 per 1 lakh people at all ages [7] and every Year 1.44-1.64 million new cases of acute stroke occur [8]. About 12.0% of strokes occur in the population aged less than 40 years [9, 10]. Prevalence of stroke in rural or in urban areas in India per every 10 lakh people was estimated to be 84-262 and 334-424 cases respectively [11].Ischemic stroke patients have been reported to have higher SUA levels compared with control subjects [12], but the association is under observation for more than a century owing to confounding’s, bias and reverse causation [13]. Several metabolic risk factors are known to alter the outcome of stroke [2, 3]. Hence, the association remains difficult to interpret and best can be described at present as controversial [14]. Few studies have shown an association between SUA and stroke related mortality [12], while another studies have yielded opposite results [15-17]. Furthermore, no ischemic stroke outcome studies have been conducted to confirm the association with stroke outcomes [18]. Therefore, studies are necessary to delineate the role SUA has in this scenario and whether it can actually be of any significance in the early prediction of individuals at risk of developing CVIS. Here we evaluated the association between SUA with the risk of mortality of CVIS patients during 72 hrs ICU stay. The results revealed that a strong correlation between SUA levels and stroke outcome in males as compared to much lesser degree in females. In some cases the elevated levels of SUA on admission lead to higher in-hospital mortality.
2. Material and Methods
2.1. Approval of Ethics Committee
- The study design and protocol was approved by the Institutional Ethical Review Committee (IERC) of Mahatma Gandhi Mission (MGM) Medical College and Hospital, Kamothe, Navi Mumbai, India. This is a case control study, conducted from June 2011 to November 2013 in MGM Hospital. Study subjects were informed of the possibility of using the data obtained for academic purpose. Confidentiality was assured to all participants and data used for this study were stripped of personally identifiable information. They were also informed about their right to withdraw their consent at any point, without any consequence to them. Patients were monitored till the end of 72 hrs ICU stay.
2.2. Patients Selection Criteria
- Study participants were in the age group of 20-75 yrs. CVIS patients (n=25) and control subjects (n=25) were selected from MGM hospital by considering strict inclusion and exclusion criteria. World Health Organization (WHO) defines stroke disturbance of cerebral function with symptoms lasting for 24 hrs or longer or leading to death with no apparent cause other than of vascular origin. We confirmed the stroke by computed tomography (CT) brain imaging or Magnetic Resonance Imaging (MRI) of brain. For comparison, age matched control subjects admitted in the ICU for reasons other than CVIS or any other serious organic illness (e.g: renal disease) were selected. Care was taken to select the control subjects, whose SUA levels were at per with the standard reference values (males ~5.0 mg/dl and females ~4.0 mg/dl [2]. Excluded were the patients with prior history of gout, chronic renal failure, liver disease, thyroid dysfunction, hematological malignancy, sepsis, neoplasms, coagulating disorders, patients with a known or possible cardiac source of embolism (atrial fibrillation, valvular heart disease), patients who were on iron or vitamin supplements or on hyperuricemic drugs like thiazide diuretics, losartan, probenecid, fenofibrate, atorvastatin, ethambutol and allopurinol. Patients whose serum creatinine levels >2 mg/dl and patients stayed less than 72 hrs in ICU, discharged from the hospital and then readmitted during the observation period were also excluded.
2.3. National Institute of Health Stroke Score (NIHSS)
- National Institute of Health Stroke Score (NIHSS) is a simple criteria developed by National Institute of Health (NIH), Bethesda, USA. This criteria was applied to clinically stratify ischemic stroke patients on admission and during 24, 48 & 72 hrs stay in ICU. Based on clinical examination of the affected patients, NIHS scale was divided into 1 to 10 scores. During 24, 48 and 72 hrs stay in ICU, we assessed the parameters like consciousness, language, gaze and head deviation, hemiparesis, facial drop, right facial palsy, right arm and right leg palsy, grip strength, ataxia, sensory, aphasia, dysarthria and agnosia etc.
2.4. Physical Examination
- The data on demographic information such as age, sex, waist circumference (WC), diabetes mellitus (DM), previous stroke history, systolic blood pressure (SBP), diastolic blood pressure (DBP), smoking and alcohol drinking etc., on admission were collected. Person who smoked at least 10 cigarettes per day for 6 months or more or the one who has smoked daily for more than 1 year or more regardless of the number of cigarettes smoked per day was considered as smoker [19]. The heights and body weights of study subjects were recorded to nearest 0.5 cm or 0.5 kg respectively. Hypertension was diagnosed when a patient had received medicine for hypertension, or had systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg after taking 5 minutes rest. Patients who used cholesterol lowering medication or had a total serum cholesterol level ≥ 200 mg/dl were classified as having hypercholesterolemia. DM was defined as fasting blood sugar of ≥126 mg/dl or history of receiving treatment for the cause. WC was measured using a flexible measuring tape and expressed the cut-offs as 102 cm for men and 88 cm for women. The currently recommended cut-offs of Body mass index (BMI) by WHO [2] include those with a BMI of 18.50-22.50 kg/m2 were classified as normal, 22.51-25.99 kg/m2 were classified as above normal and those with a BMI 26-29.99 kg/m2 were defined as overweight and those with BMI >30 kg/m2 were defined as obese. Hypertriglyceridemia was defined as triglyceride levels ≥150 mg/dl or specific medications; Low HDL-c levels defined as <40 mg/dl for male and < 50 mg/dl for female; Hypertension was defined as BP ≥ 130 mm systolic or ≥ 85 mm diastolic.
2.5. Blood Collection and Determination of Serum Biochemical Profiles
- Venous blood sampled in 5 ml sterile vacutainers on admission and during 24, 48 and 72 hrs post admission. Plasma was separated by centrifugation at 1000 rpm for 10 min, and used for the determination of various biochemical parameters. Serum levels of UA were estimated on admission and during 72 hrs ICU stay by Uricase method [20]. The levels of triglycerides (TG’s) [21], total cholesterol (TC) [22], Low-density lipoprotein cholesterol (LDL-c), high density lipoprotein cholesterol (HDL-c) [23] and creatinine [24] were determined using the methods given in the parentheses. The levels of LDL-c were calculated using the method described earlier [25].
2.6. Statistical Analysis
- Data was analyzed using Graph Pad Prism software version 5.0 (Graph Pad software, USA). Basic data on SUA, SBP and DBP, total cholesterol, LDL-c, HDL-c, triglycerides and creatinine were expressed as mean ± Standard Deviation (SD). Kaplan–Meier analysis was used to assess all-cause mortality based on SUA levels using quartiles (Q1-Q4). Traditional risk factors for stroke considered for adjustment include age, gender, smoking, hypertension and stroke, weight, BMI, WC, fasting glucose, LDL-c, HDL-c and triglycerides. The association of SUA levels with trends across quartiles and all-cause of mortality was analyzed by multivariate Cox proportional hazards model [26]. To determine the contribution of using SUA as a predictive marker, we calculated the concordance indices [27] with and without SUA. The improvement in predictability with the addition of SUA to the risk factors was assessed by the difference in the concordance indices [28]. The difference of the concordance indices was bias corrected and bootstrapping was utilized to generate 95% confidence intervals (CI). The significance of statistical verification was set at P < 0.05.
3. Results
3.1. Patients Admission Characteristics
- The study consists of 25 age matched control subjects to 25 CVIS patients admitted in the Department of Internal Medicine of MGM hospital, Navi Mumbai. The data on demographic information like age, sex, SBP, DBP, status of diabetes, previous stroke, previous and current history of smoking, alcohol drinking, fever, headache, dyspnea, nausea and vomiting etc, were collected on admission and during 72 hrs ICU stay.
3.2. Distribution of Study Subjects Based on the Age
- The mean age of the control subjects (n=25) was 61.43. ± 12.01, which matched with CVIS patents, 63.21± 13.16. The control subjects were in the age range between 20-39 yrs (2 males &1 female), 40-59 yrs (5 males & 4 females) and over 60 yrs (9 males & 4 females). Whereas CVIS patients were in the age range of 20-39 yrs (2 males & 1 female), 40-59 yrs (6 males & 4 females) and over 60 yrs (8 males & 4 females).
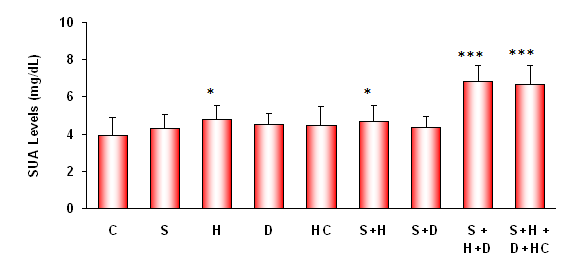 | Figure 1. Association between SUA levels and various risk factors in stroke patients and compared with the mean values of control subjects on admission. SUA levels showed significant correlation with hypertension (H) and other stroke risk factors associated with it as indicated (*: P<0.05;***P<0.001). (C: Control; S: Smoking; H: Hypertension; D: Diabetes and HC: Hypercholesterolemia) |
3.3. Demographic Characteristics of Study Subjects
- CVIS patients comprising 28% hypertensives-H (n=7), 8% diabetics-D (n=2), 24% smokers-S (n=6), 16% hypercholesterolemia-HC (n=4); 12% hypertensives + smokers-H+S (n=3), 8% hypertensives + diabetes + smokers H+D+S (n=2), 4% hypertention + diabetics + smokers + hypercholesterolemia H+D+S+HC (n=1). SUA levels were determined in all the study subjects, and observed a significant increase (P<0.001) in CVIS patients presented with hypertension and other risk factors associated with stroke. SBP was found to be significantly high in CVIS patients as compared to control subjects (159 ± 5.08 Vs 120±4.78 mm/Hg). In contrast, DBP was significantly lower in CVIS patients than the control subjects (81.09±3.07 Vs 73.00 ± 4.84 mm/Hg) (Table-1). Their past history showed none of the study subjects suffered with either renal or liver diseases. A significant negative correlation was observed between the age and SUA levels.
3.4. Distribution of Study Subjects Based on Body Weight
- Control subjects were in the weight ranges between 40-59 kgs (2 males & 3 females), 60-79 (7 males & 5 females), 80-99 kgs (6 males & 1 female) and > 100 kgs (1 male & 0 female). Whereas CVIS patients were in the weight ranges between 40-59 kgs (2 males & 2 females), 60-79 kgs (8 males & 5 females), 80-99 kgs (5 males & 2 females) and >100 kgs (1 male & 0 female). The results indicated that, SUA levels were significantly high in CVIS patients who were in the weight range of 60-79 kgs and >100 kgs than the control subjects of similar body weight range (Data not showed).
3.5. Distribution of Study Subjects Based on BMI (kg/m2)
- Of the 25 control subjects, 20% (2 males & 3 females), 48% (7 males & 5 females), 28% (6 males & 1 females) and 4% (1 male & 0 female) were in the BMI ranges between 18.50-22.50 kg/m2, 22.51-25.99 kg/m2, 26-29.90 kg/m2 and >30 kg/m2 respectively. Of the 25 CVIS cases, 16% (2 males & 2 females), 40% (6 males & 4 females), 28% (5 males & 2 females), and 16% (3 males & 1 females) were in the ranges between 18.50-22.50 kg/m2, 22.51-25.99 kg/m2, 26-29.90 kg/m2 and >30 kg/m2 respectively. CVIS patients had 13.64% higher WC than the control subjects (101.30 ±12.37 cm Vs 89.14±6.43 cm). In CVIS patients with higher BMI, the levels of SUA significantly (P<0.001) high. We observed a positive correlation of SUA with BMI and WC.
3.6. Comparison of SUA Levels between Males and Females on Admission
- To know whether SUA levels varies between males and females and the significance of any changes in the early prognosis of stroke. On admission, males (CVIS cases & control subjects) showed 28.21% higher levels of SUA than the females (5.50 ± 0.77 Vs 4.29 ± 0.85 mg/dl). CVIS males (n=16) had 45.90% higher SUA levels than control males (n=16) (6.58±0.89 Vs 4.51±0.66). CVIS females (n=9) had 23.12% higher SUA levels than control females (n=9) (4.74±0.79 Vs 3.85±0.59 mg/dl) (Figure 2a &b).
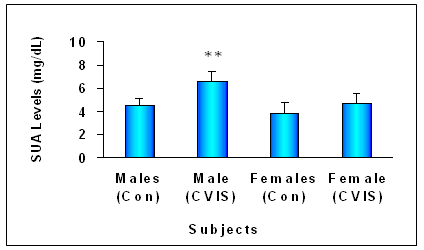 | Figure 2a. Levels of SUA in control subjects and CVIS patients on admission. CVIS males and females showed significantly higher SUA levels than control males and females. Each value is the mean ± S.D (**=P<0.01) |
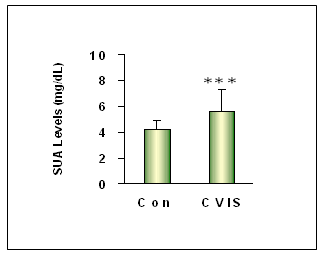 | Figure 2b. Levels of SUA in control subjects and CVIS patients on admission. Mean levels of SUA in control subjects and CVIS patients. SUA levels were significantly elevated in CVIS patients. Each value is the mean ± S.D (***=P<0.001) |
3.7. Biochemical Characteristics of Study Subjects
- Serum biochemical profiles such as blood glucose (mg/dl), serum triglycerides (mg/dl), total cholesterol (mg/dl), LDL-c (mg/dl), HDL-c (mg/dl), LDL-c/HDL-c ratio, total cholesterol/HDL-c ratio, SUA (mg/dl) and creatinine (mg/dl) levels were determined (Table-1). The results indicated that except HDL-c and creatinine all other parameters were significantly elevated in CVIS patients as compared to control subjects.
3.8. Association of SUA Levels and Morbidity/Mortality of CVIS Patients
- Next, we evaluated the extent of changes in SUA levels and to correlate the changes with morbidity/mortality of CVIS patients. The results indicated that during 72 hrs of ICU stay, the levels of SUA in some of the CVIS cases were significantly (P<0.001) increased compared to the admission day. The average levels of SUA in CVIS patients at admission was higher by 35.41% than control subjects (5.66± 1.05 Vs 4.18± 0.76 mg/dl). The mean SUA levels were increased by 15.37, 32.51 and 56.18% respectively by the end of 24, 48 and 72 hrs ICU stay compared to the admission day. In CVIS males by the end of 72 hrs ICU stay, the percent increase of SUA levels were found to be 5.78, 18.09 and 30.40 respectively compared to the admission day. Similarly, the percent increase in SUA levels in CVIS female patients by the end of 72 hrs ICU stay was found to be elevated by 1.48, 9.07 and 18.20 respectively compared to the admission day. SUA predicted mortality in males better than females as analyzed by multivariate Cox model (Hazard Ratio (HR) = 1.07, 95% CI = 1.12-1.51, P = 0.03 in men; HR = 1.45, 95% CI = 1.27–1.90, P = 0.001 in women). Variables considered for adjustment in a multivariate Cox model included age, gender, WC, DM, body weight, alcohol, smoking, history of hypertension, BMI, LDL-c, HDL-c, triglycerides and fasting plasma glucose. After adjusting for all these variables, a relationship between SUA level and all-cause mortality was maintained (HR = 1.47, 95% CI = 1.33–1.69, P = 0.001). Separate multivariate analysis that included age, gender, current smoking, HDL-c, SBP, total cholesterol, hypertension, WC, DM and triglycerides were created with and without SUA to determine if the addition of SUA to factors that are associated with mortality increased the prediction of the analysis. The concordance index for the analysis including SUA was 0.81; excluding SUA rendered a concordance index of 0.90. The bias corrected difference was 0.051 (95% CI, 0.05-0.107, P = 0.03). This indicates that adding UA parameter significantly improves the predictive accuracy of the model for mortality. Five CVIS male patients who had SUA levels as high as 8.33, 7.47, 7.80, 5.67 and 5.89 mg/dl and two female CVIS patient who had high SUA levels 5.45 and 5.65 mg/dl were succumbed during ICU stay.
3.9. Distribution of Study Subjects in Different Quartiles Based on SUA Levels
- Study subjects were divided into Q1-Q4 quartiles based on their SUA levels. Patients in higher quartiles more often had hypertension, smoking, DM, high LDL-c levels with elevated SUA levels. Patients who are in quartile 3/4 showed higher ICU mortality. Five out of six stroke males whose SUA levels were as high as 8.33, 7.47, 7.80, 5.67 and 5.89 mg/dl and two female patients whose SUA levels were recorded as 5.45 and 5.65 mg/dl) were belonged to quartile-Q3/Q4, and these 7 patients were died. Of the 25 patients, 7 patients died and remaining 18 patients were discharged from the hospital (Fig-3).
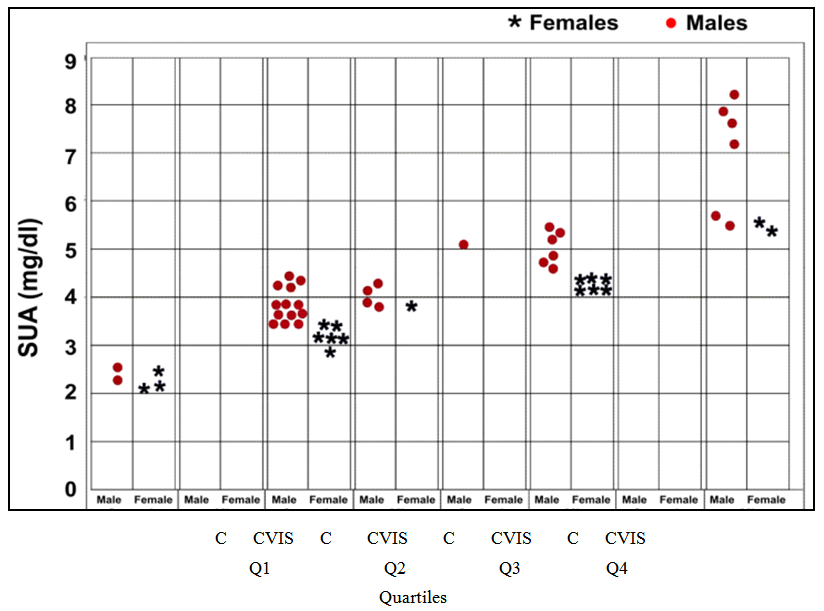 | Figure 3. Survival of CVIS patients stratified by SUA quartiles (Q1–Q4) at baseline. The mean levels of SUA in control male subjects were 4.51 ± 0.90 mg/dl mg/dl, whereas in females the mean levels were 3.85 ± 0.92 mg/dl. The mean levels of SUA in male CVIS cases was 5.55 ± 0.89 mg/dl and 4.30 ± 0.81 mg/dl in females ( = Females; = Females;  = Males). (C=Control subjects) = Males). (C=Control subjects) |
4. Discussion
- India like other developing countries is in the midst of a ischemic stroke epidemic. Stroke is becoming an important cause of premature death and disability in low-income and middle-income countries [11]. Currently, the literature features controversial reports on the relationship between SUA levels and acute cerebrovascular ischemic stroke (CVIS) (29-31). Studying the role of SUA in the pathogenesis of acute CVIS has been challenging because of various metabolic risk factors associated with this disorder. The available epidemiological and case-control studies have indicated that Indians are highly susceptible to diabetes and cardiovascular disease (CVD) even with only modest overweight [32-34]. Therefore, the importance of accurate prognostication during the care of patients with stroke is being increasingly recognized. In this study, using a well standard neurological NIHS Score, we identified stroke patients and correlated the results with the severity of this disorder. Kaplan–Meier analysis was used to assess all-cause mortality based on SUA levels using quartiles on admission and during 24 hrs, 48 hrs and 72 hrs ICU stay. Our results demonstrated that SUA levels were more in males than in females, attributing high risk of ischemic stroke in former than the later. This could also be explained as due to the protective action of estrogen upto menopause stage [35, 36] or gender significance as majority of the women who presented with CVIS were postmenopausal (6 out of 8 patients). Similar results were reported earlier [37-39]. Next, we found increased baseline levels of SUA associated with several metabolic risk factors including hypertension, diabetes, smoking, and hypercholesterolemia, elevated levels of BMI, triglycerides, total cholesterol and LDL-c. Therefore we reasoned it is difficult to establish whether or not an independent association between SUA and CVIS exists. In this regard, our data strongly suggests that increasing baseline SUA levels in CVIS patients positively correlated with these risk factors. This is not unexpected given the knowledge about the significance of hyperuricemia as a marker of abnormal oxidative metabolism. This finding is in line with previous report from a large cohort study, National Health and Nutrition Examination Survey-III, USA, which says an independent and significant association between elevated UA with CVIS [40]. BMI is risk factor for both hyperuricaemia and BP [41]. Milionis et al [38] reported significantly higher levels of SUA among overweight patients (BMI > 25) as compared to non-obese patients. Further, these authors reported a positive correlation between SUA and smoking as compared to non-smokers. Elevated glucose levels known to disrupt the blood-brain barrier [42] and promote stroke [43]. On admission, we observed 13% more of serum glucose levels in CVIS patients than the control subjects, suggesting the involvement of glucose with increased risk of stroke. In a recent study, Patil et al [44] demonstrated patients who presented with acute stroke had higher SUA levels and remarkably low HDL-c. Bonora et al [45] studied 957 young men and reported a significant positive correlation between SUA and triglyceride, cholesterol and LDL-c levels. In another study by Mozos et al [46] elevated SUA is associated with increased burden of cerebral ischemic pathology in older adults. Conversely, Chamorro et al [47] reported elevated SUA level may be neuro-protective in patients with ischemic stroke. An explanation to this comes from studies [48, 49], which showed SUA may work as a pro-oxidant under certain circumstances, particularly if the levels of other antioxidants including vitamin-C and E, glutathione, L-carnithine, coenzyme-Q, melatonin, alpha lipoic acid, bilirubin and ascorbate are low .The present study does not address the mechanisms by which SUA can associate with stroke related mortality. However, the available literature revealed that, SUA is known to contribute to endothelial dysfunction by impairing nitric oxide (NO) production. SUA has been shown to be inversely correlated with the measures of functional capacity and maximal oxygen intake [50]. Therefore, we believe that elevated SUA levels in stroke patients may lead to endothelial damage and increased vascular permeability. Another putative mechanism likely to be involved is association with xanthine oxidase (XO) and cyclooxygenase systems with ischemic stroke in the presence of elevated SUA levels [51], which cause neuronal death [52]. The action of XO leads to generation of superoxide radical anions and the reactive oxygen species (ROS) as supported by the fact that knockout mice for mitochondrial superoxide dismutase (mSOD) genes display larger brain lesions after focal ischemia [53]. The increased levels of ROS can make the brain more susceptible to oxidative stress [54]. At present, it is unclear whether high SUA levels promote or protect against the development of CVIS, or simply act as a passive marker of increased risk in stroke patients. Using NIHS Score and Kaplan–Meier quartile estimates, stroke severity and outcomes were determined and observed a significant correlation between higher NIHS score (NIHSS-9) and SUA levels in stroke patients assessed on admission and during ICU stay for 24, 48 and 72 hrs. When assessed on admission, most of the patients had SUA levels ~5.0 mg/dl, but in some cases the levels were increased during 24, 48 and 72 hrs stay in ICU. Fourteen patients were presented with NIHS Score-9. Of these, 7 patients (5 males & 2 females) were died by the end of 72 hrs ICU stay. Of these 5 male patiens (NIHS Score-9), one patient showed SUA levels as high as 8.33 mg/dl. Two female stroke patients who also died showed elevated SUA levels (>5 mg/dl), attributing increased SUA levels during ICU may be associated with higher morbidity/mortality. Our findings are in concordant with the report of Karagiannis et al [19] showing an independent relationship between higher SUA levels on admission and in-hospital mortality as compared to those who were discharged.Kaplan–Meier estimates of morbidity/mortality showed gender variation across SUA quartile. The CVIS patients who were in upper quartile were succumbed as compared to those in the lowest quartile. We observed 12 patients (6 males & 6 females) in the quartile-Q3 and 8 patients (6 males & 2 females) in quartile-Q4 had significantly higher levels of SUA (NIHS score-9), suggesting patients with higher SUA levels may suffer more severe ischemia stroke. In the unadjusted Cox regression analysis, for about 1 mg/dl elevation in the SUA, the risk of death from all causes increased by 23% (95% CI, 1.09 –1.55, P = 0.001). In conclusion, our results suggest that elevated SUA is an independent predictor for good clinical outcome of acute stroke patients regardless of CVIS risk factors. Our study highlighting the importance of identifying individuals with high SUA levels. This would allow better risk stratification of patients at the time of initial diagnosis or during hospital stay. The mechanisms through which hyperuricemia increases the risk of stroke is the current focus of our research. If the current findings stand the test of time, one of the causes of CVIS may be suggested and further investigated.
4.1. Strengths and Limitations of the Study
- Few limitations existed which should be taken into account when interpreting the results. Among the limitations, the fact is that this is not a population-based study. It is a single-center case control study restricted to a local hospital, based on the data collected from a small number of ethnically homogeneous, unselected clinically assessed CVIS patients, therefore may not accurately reflect the majority of other population. Also we believe that, the greater risk of acute stroke events or morbidity/mortality attributable to hyperuricemia in our study challenges the antioxidant properties shown by UA. The results obtained in our study does not provide support for the view that the antioxidant capacity of UA that ameliorates the clinical prognosis of patients with CVIS. The practical application of this conclusion deserves some thought. Notwithstanding these limitations, we consider that our study makes an important contribution, since similar studies are few and their results are inconsistent (29, 31).
5. Conclusions and Clinical Implications
- The relationship of SUA to acute CVIS is controversial. The present results revealed that: (1) SUA levels were significantly higher in men than women and showed good correlation with males than females, (2) Strong association of SUA was found with stroke in males compared with much lesser degree in females, (3) A significant negative correlation was observed between the age of CVIS patients and their SUA levels, (4) Baseline SUA is independently associated with either CVIS mortality or all cause death after adjustment for other metabolic risk factors and (5) SUA may become a reliable biochemical surrogate marker or proxy of acute stroke outcome in general populations. The above findings have significant clinical implications, given the fact that CVIS has been a growing public health burden across the world. Early identification of stroke by developing additional cost-effective biomarkers will be of immense value for early and prompt treatment or prevention measures can be started and huge hidden burden of future CVIS complications can be reduced. In addition to this, controlling hyperuricemia might also be a promising strategy for the prevention of CVIS. Taking into account, all the limitations of the study and the previous literature on the prevalence of CVIS, we strongly recommend that patients with high levels of SUA on admission should be closely monitored for CVIS associated complications. In support of our current findings, further studies are necessary to confirm these results with a larger group of patients from many different areas/regions. Contributors: Conceived and coordinated the investigation: JG. Performed the experiments, preparation and analysis of data: CRKV. Wrote the manuscript: JG and CRKV.Conflict of interest: The authors have no conflicts of interest to declare.
ACKNOWLEDGEMENTS
- We acknowledge all the patients who gave their consent to be the part of this study. We are thankful to the entire staff of Internal Medicine OPD & ICU staff of MGM Medical College and Hospital for their consistent support and help. We appreciate Dr. A. R. Passi for assistance in the statistical analysis.
References
| [1] | Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. The Lancet Neurology. 1997; 349: 1269-76. |
| [2] | WHO Expert Consultation. Appropriate body-mass index in Asian populations and its implications for policy and intervention strategies. Lancet. 2004; 363: 157-63. |
| [3] | Grysiewicz RA, Thomas K, Pandey DK. Epidemiology of Ischemicand Hemorrhagic Stroke: Incidence, Prevalence, Mortality, and Risk Factors. Neurol Clin. 2008; 26: 871-95. |
| [4] | Bath P, Lees K. ABC of arterial and venous disease. Acute stroke. BMJ. 2000; 320:920- 23. |
| [5] | Mariani E, Polidori MC, Cherubini A, et al. Oxidative stress in brain aging, neurodegenerative and vascular diseases: An overview. J Chromat . 2005; 827:65-70. |
| [6] | Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics-2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009; 119:e21–181. |
| [7] | Dalal P, Bhattacharjee M, Vairale J, Bhat P. Mumbai stroke registry (2005-6)-Surveillance using WHO STEPS stroke instrument: challenges and opportunities. JAPI. 2008; 56: 675-79. |
| [8] | Murthy J. Thrombolysis for stroke in India: Miles to go. Neurology India. 2007; 55: 3-5. |
| [9] | Bansal BC, Prakash C, Jain AL, Brahmanandam KR. Cerebrovascular disease in young individuals below the age of 40 years. Neurol India. 1973; 21:11-18. |
| [10] | Shah B, Mathur P. Workshop Report on Stroke Surveil lance in India. Division of Non communicable Diseases, Indian Council of Medical Research, New Delhi, 2006. |
| [11] | Pandian JD, Sudhanb P. Stroke Epidemiology and Stroke Care Services in India. J Stroke. 2013; 15: 128-34. |
| [12] | Hozawa A, Folsom AR, Ibrahim H, Javier Nieto F, Rosamond WD, Shahar E. Serum uric acid and risk of ischemic stroke: the ARIC Study. Atherosclerosis 2006; 187:401-07. |
| [13] | Barbosa MCC, Brandao AA, Pozzan R, Magalhães MEC, Campana EMG, Fonseca FL, Pizzi OL, de Freitas EV and Brandão AP. Association between uric acid and cardiovascular risk variables in a non-hospitalized population. Arq Bras Cardiol. 2011; 96: 96: 212-218. |
| [14] | Bowman GL, Shannon J, Frei B, Kaye JA, Quinn JF. Uric acid as a CNS antioxidant. J Alzheimers Dis. 2010; 19: 1331-1336. |
| [15] | Uemura Y, Miller JM, Matson WR, Beal MF. Neurochemical analysis of focal ischemia in rats. Stroke. 1991; 22:1548-53. |
| [16] | Imarhiagbe FA and Idemudia JO. Serum uric acid and acute stroke outcome in Nigerian Africans. Annals of Nigerian Medicine.2013; 6: 75-79. |
| [17] | Weir CJ, Muir SW and Walters MR, Lees KR. Serum Urate as an independent predictor of poor outcome and future vascular events after acute ischemic stroke. Stroke. 2003; 34: 1951-56. |
| [18] | Zhang B, Cong Gao C,Yang N, Zhang WZ, Song XW, Yin JR, Pu SX, Yi YH and Gao QC. Is elevated SUA associated with a worse outcome in young Chinese patients with acute cerebral ischemic stroke? BMC Neurology. 2010; 10:82- 87. |
| [19] | Karagiannis A, Mikhailidis DP, Tziomalos K, Sileli M et al. Serum uric acid as an independent predictor of early death after acute stroke. Circ J.2007; 71:1120-27. |
| [20] | Fossati P, Prencipe L, Bari G. Use of 3,5-dichloro-2- hydroxybenzenesulfonic acid/4-aminophenazonechromogenic system in direct enzymic assay of uric acid in serum and urine. Clin Chem. 1980; 26: 227-31. |
| [21] | Jungner I, Walldius G, Holme I, Kolar W, Steiner E. Apolipoprotein B and A-1 in relation to serum cholesterol and triglycerides in 43000 Swedish males and females. Int J Clin Lab Res. 1992; 21: 247-55. |
| [22] | Siedel J, Schlumberger H, Klose S, Ziegenhorn J, Wahlefeld AW. Improved reagent for the enzymatic determination of serum cholesterol. J Clin Chem Clin Biochem. 1981; 19: 838-39. |
| [23] | Kostner G. Enzymatic determination of cholesterol in high density lipoprotein fractions prepared by polyanion precipitation. Clin Chem. Letter.1976; 22:69. |
| [24] | Owen JA, Iggo B, Scandrett FJ and Stewart CP. The determination of creatinine in plasma or serum and in urine: a critical examination. Biochem J. 1954; 58: 426-37. |
| [25] | Friedewald WT, Levy RI, Fredrickson DS. Clin Chem. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. 1972; 18: 499-502. |
| [26] | Cox DR. Regression models and life-tables. J R Stat Soc. 1972; 34:187 |
| [27] | Harrell FE Jr, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. J Am Med Assoc 1982; 247: 2543 – 2546. |
| [28] | Kattan MW. Evaluating a new marker’s predictive contribution. Clin Cancer Res 2004; 10: 822– 824. |
| [29] | Hozawa A, Folsom AR, Ibrahim H, et al. Serum uric acid and risk of ischemic stroke: the ARIC Study. Atherosclerosis 2006; 187:401–7. |
| [30] | Charan Reddy KV, Mishra G, Jaishree G. Serum Uric Acid as Cardiovascular Disease Marker: Premises and Promises. Int J Sciences. 2014b; 3:18-26. |
| [31] | Weir CJ, Muir SW, Walters MR, Lees KR. Serum urate as an independent predictor of poor outcome and future vascular events after acute stroke. Stroke 2003; 34:1951-6. |
| [32] | WHO. Burden of Disease Statistics. Geneva, Switzer- land: World Health Organization. Available: http:// www. who.int / healthinfo /bod / en / index . Html, 2002. |
| [33] | Charan Reddy KV and Jaishree G. Prognostic role of serum uric acid in critically ill patients with non-ST elevation acute myocardial infarction. J Cardiov dise. 2014 (in press). |
| [34] | Charan Reddy KV, Nikhila AP and Jaishree G. Association of Fasting Blood Glucose with Cardiovascular Risk Factors:Results From a Semi-Urban Population Based Study on 1502 subjects. J Clin Res Letters. 2014; 5: 70-76. |
| [35] | Saltiki K, Cimponeriu A, Lili K, Peppa M, Anastasiou E, Alevizaki M. Severity of coronary artery disease in postmenopausal diabetic women. Hormones 2008; 7:148-55. |
| [36] | Scott E, Zhang QG, Wang R, Vadlamudi R, Brann D. Estrogen neuroprotection and the critical period hypothesis. Front Neuroendocrinol. 2012; 33: 85-104. |
| [37] | Pearce J, Aziz H. Uric acid and plasma lipids in cerebrovascular disease. I. Prevalence of hyperuricaemia. Br Med J.1969; 4:78-80. |
| [38] | Milionis HJ, Kalantzi KJ, Goudevenos JA, Seferiadis K, Mikhailidis DP, Elisaf MS. Serum uric acid levels and risk for acute ischaemic non-embolic stroke in elderly subjects. J Intern Med. 2005; 258: 435-441. |
| [39] | Culleton BF, Larson MG, Kannel WB, Levy D. Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med. 1999;131:7-13. |
| [40] | Yanyan Z, Bhavik P and Hyon C. Prevalence of Hyperuricemia in the US General Population: The National Health and Nutrition Examination Survey (NHANES) 1999-2008. Arthritis Rheumatism. 2010; 62 (Suppl 10):1363. |
| [41] | Strasak AM, Strasak AM , Kelleher CC, et al. Serum uric acid is an independent predictor for all major forms of cardiovascular death in 28,613 elderly women: a prospective 21-year follow-up study. Int J Cardiol. 2008; 10: 125: 232-239. |
| [42] | Dietrich WD, Alonso O, Busto R. Moderate hyperglycemia worsens acute blood-brain barrier injury after forebrain ischemia in rats. Stroke.1993; 24: 111–6. |
| [43] | DeCourten-Myers GM, Kleinholz M, Holm P, DeVoe G, Schmitt G, et al. Hemorrhagic infarct conversion in experimental stroke. Ann Emerg Med.1992; 21:121–6. |
| [44] | Patil TB, Pasari AS , Sargar KM, Vinayak E. Shegokar VE, Bansod YV, Patil MB. Serum Uric Acid Levels in Acute Ischemic Stroke: A Study of 100 Patients. J Neurol Res. 2011; 1:193-200. |
| [45] | Bonora E, Targher G, Zenere MB, Saggiani F, et al. Relationship of uric acid concentration to cardiovascular risk factors in young men. Role of obesity and central fat distribution. The Verona Young Men Atherosclerosis Risk Factors Study. Int J Obes Relat Metab Disord. 1996; 20:975-80. |
| [46] | Mozos I, Chiulana C, Goruna C, Costeab S. Serum uric acid in stroke. Annals of West University of Timisoara, Series of Chemistry. 2007; 16: 227- 36. |
| [47] | Chamorro A, Obach V, Cervera A, Revilla M, Deulofeu R, Aponte JH: Prognostic significance of uric acid serum concentration in patients with acute ischemic stroke. Stroke.2002; 33:1048-52. |
| [48] | Davies KJA, Sevanian A, Muakkassah-Kelly SF, Hochstein P. Uric acid iron ion complexes. A new aspect of the antioxidant functions of uric acid. Biochem J 1986; 235: 747–54. |
| [49] | Miller NJ, Rice-Evans C, Davies MJ, Gopinathan V, Milner A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. 1993; Clin Sci. 84: 407-12. |
| [50] | Krishnan E. Hyperuricemia and Incident Heart Failure. Circ Heart Fail. 2009; 2: 556-562. |
| [51] | Piantadosi CA, Zhang J. Mitochondrial generation of reactive oxygen species after brain ischemia in the rat. Stroke. 1996; 27:327–32. |
| [52] | Alexandrova M, Bochev P, Markova V, et al. Dynamics of free radical processes in acute ischemic stroke: influence on neurological status and outcome. J Clin Neurosci. 2004; 11:501–6. |
| [53] | Murakami K, Kondo T, Kawasw Y, et al. Mitochondrial susceptibility to oxidative stress exacerbates cerebral infarction that follows permanent focal cerebral ischemia in mutant mice with manganese superoxide dismutase deficiency. J Neurosci. 1998; 18:205-13. |
| [54] | Badiger S, Akkasaligar PT, Narone U. Hyperglycemia and Stroke. International Journal of Stroke Research .2013; 1: 1-6. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML