-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Stroke Research
2014; 2(1): 1-5
doi:10.5923/j.stroke.20140201.01
Intimal Fibromuscular Dysplasia of the Carotid Artery: Case Report and Review
Juan Nader-Kawachi1, Ernesto Anaya-Santacruz2, Carlos Peñaherrera-Oviedo1, María de la Luz Andrade-Magdalen1, Luis Felipe Alva3, Fredy Chable-Montero4
1Neurology Department, Fundación Clínica Médica Sur, México DF
2Vascular Surgery Department, Fundación Clínica Médica Sur, México DF
3Image Department, Fundación Clínica Médica Sur, México DF
4Pathology Department, Fundación Clínica Médica Sur, México DF
Correspondence to: Juan Nader-Kawachi, Neurology Department, Fundación Clínica Médica Sur, México DF.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Differential diagnosis of stroke in young patients includes fibromuscular dysplasia (FMD). As renal arteries are the most frequent target in this disease, early-onset high blood pressure appears as the most common manifestation. Craniocervical vessel disease is less known and often asymptomatic, debuting with ischemic or hemorrhagic stroke. Three histological subtypes of the disease exist, depending on it affecting the tunica media, intima or adventitia of the artery. Stroke is commonly associated with FMD of the middle layer. Cases of intimal FMD are infrequent and there are fewer than 15 reports. We present a case whose diagnosis was fibromuscular dysplasia of the carotid artery intima and provide a review of the available information on the disease.
Keywords: Fibromuscular Dysplasia, Carotid Artery, Ischaemic Stroke, Vascular Evens, Endarterectomy, Intimal Fibroplasia
Cite this paper: Juan Nader-Kawachi, Ernesto Anaya-Santacruz, Carlos Peñaherrera-Oviedo, María de la Luz Andrade-Magdalen, Luis Felipe Alva, Fredy Chable-Montero, Intimal Fibromuscular Dysplasia of the Carotid Artery: Case Report and Review, International Journal of Stroke Research, Vol. 2 No. 1, 2014, pp. 1-5. doi: 10.5923/j.stroke.20140201.01.
1. Introduction
- Fibromuscular dysplasia (FMD) is an idiopathic, multifocal, non-inflammatory and non-atherosclerotic process affecting small and medium-sized arteries through the body, without compromise of veins or lymphatics[1-3]. The disease has an incidence of 0.6 to 1.1% in the United States, being three times more frequent in women aged 25 to 50 years, and in Caucasians[4, 5]. The most commonly affected vessels are the renal arteries in 60-70% of cases, followed by cervicocephalic arteries (25-30%). Other vascular systems less frequently involved are the visceral and coronary arteries, and those of the extremities[3, 5, 6]. Histopathological classification of the disease depends on the layer of the artery wall that is affected[7, 8]. Renal FMD presents with arterial hypertension, while cervical artery disease is often asymptomatic[4, 5, 9]. However, some patients with symptomatic carotid FMD may develop a stroke, and this most often occurs in young patients without other risk factors[10]. The RENAMEVASC study, one of the most recent epidemiological reports on stroke in a Latin American population, showed that 1.4% of ischemic events, and 1.3% of hemorrhages were caused bynon-atherosclerotic vascular disease, a group of entities that includes arterial dissection and, less commonly, FMD[11, 12]. Nevertheless, there is no reliable information on stroke incidence related to FMD. There are less than 15 histologically confirmed cases of intimal FMD presenting with ischemic stroke. Here, we report a patient with multiple simultaneous ischemic strokes related to carotid artery-to-artery embolism, in whom intimal FMD was histologically diagnosed.
2. Case Presentation
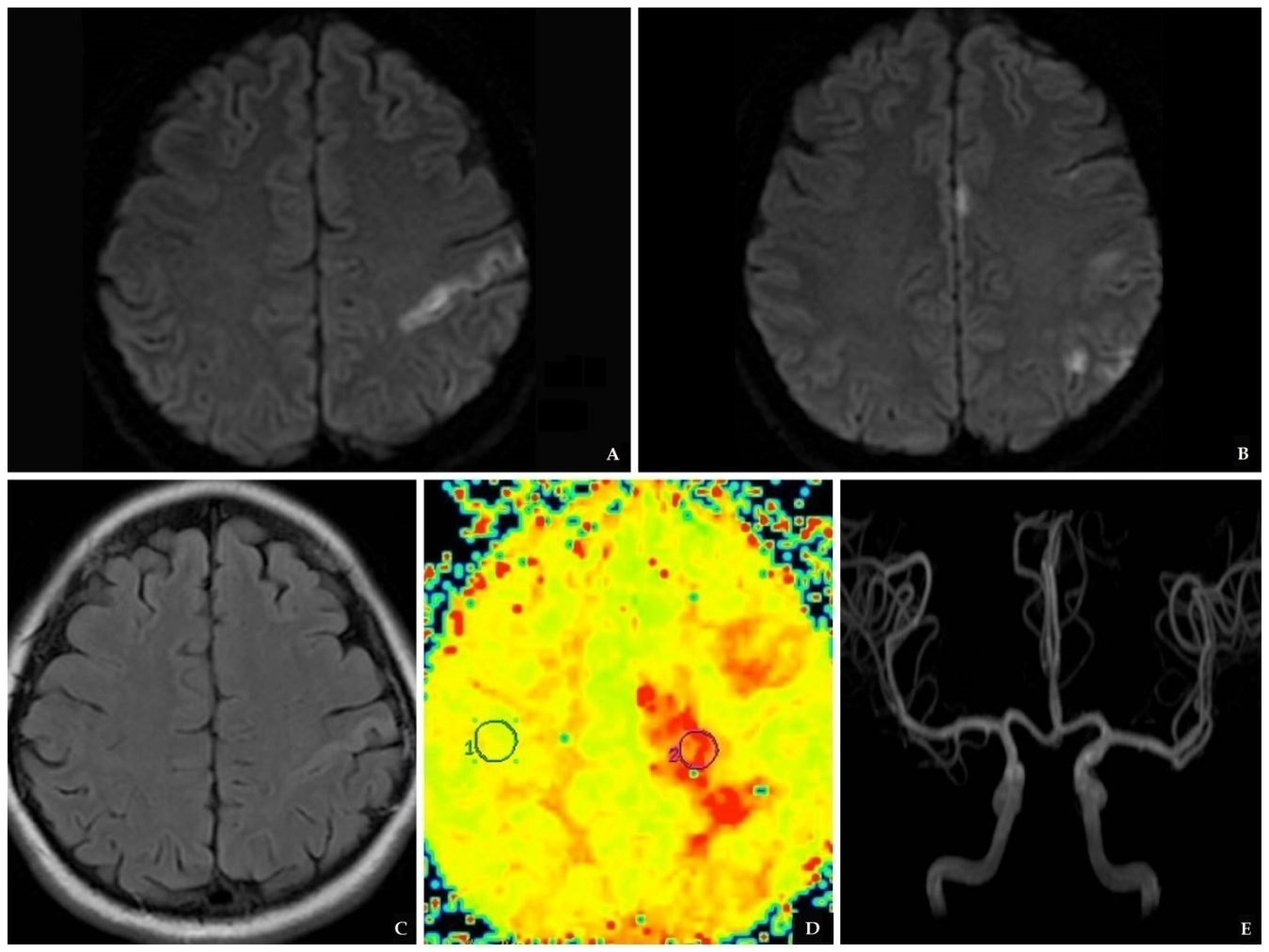
- A 40 year old, previously healthy woman was evaluated 30 minutes after the sudden onset of confusion and paraphasia, followed by paresis and paresthesia of right extremities, predominantly on the upper limb, and tonic movements of the left upper extremity. When attempting to stand up, she lost balance and fell. Ten minutes later, she was found to have confused language, paraphasias, and spasticity in her right limbs. She arrived to the ER with normal vital signs. On initial assessment, she had 15 points in the Glasgow Coma Scale and 4 points on NIHSS[13]. Neurological examination was remarkable for slight confusion but no altered speech, right-side paresis with increased stretch reflexes and ipsilateral hypoesthesia. A head CT was normal. Thrombolysis was not considered as she had a low NIHSS grade and she started to improve soon after admission Diffusion-weighted MRI revealed an area of cortico-subcortical hyperintensity in the left precentral gyrus, and three small hyperintense lesions in the ipsilateral frontal and supramarginal cortex and cingulate gyrus; these lesions were also visible on FLAIR and were consistent with ischemia. Perfusion weighted image showed ischemic penumbra in the left semioval center, and MRA showed no alterations in the intracranial vasculature (Picture 1).
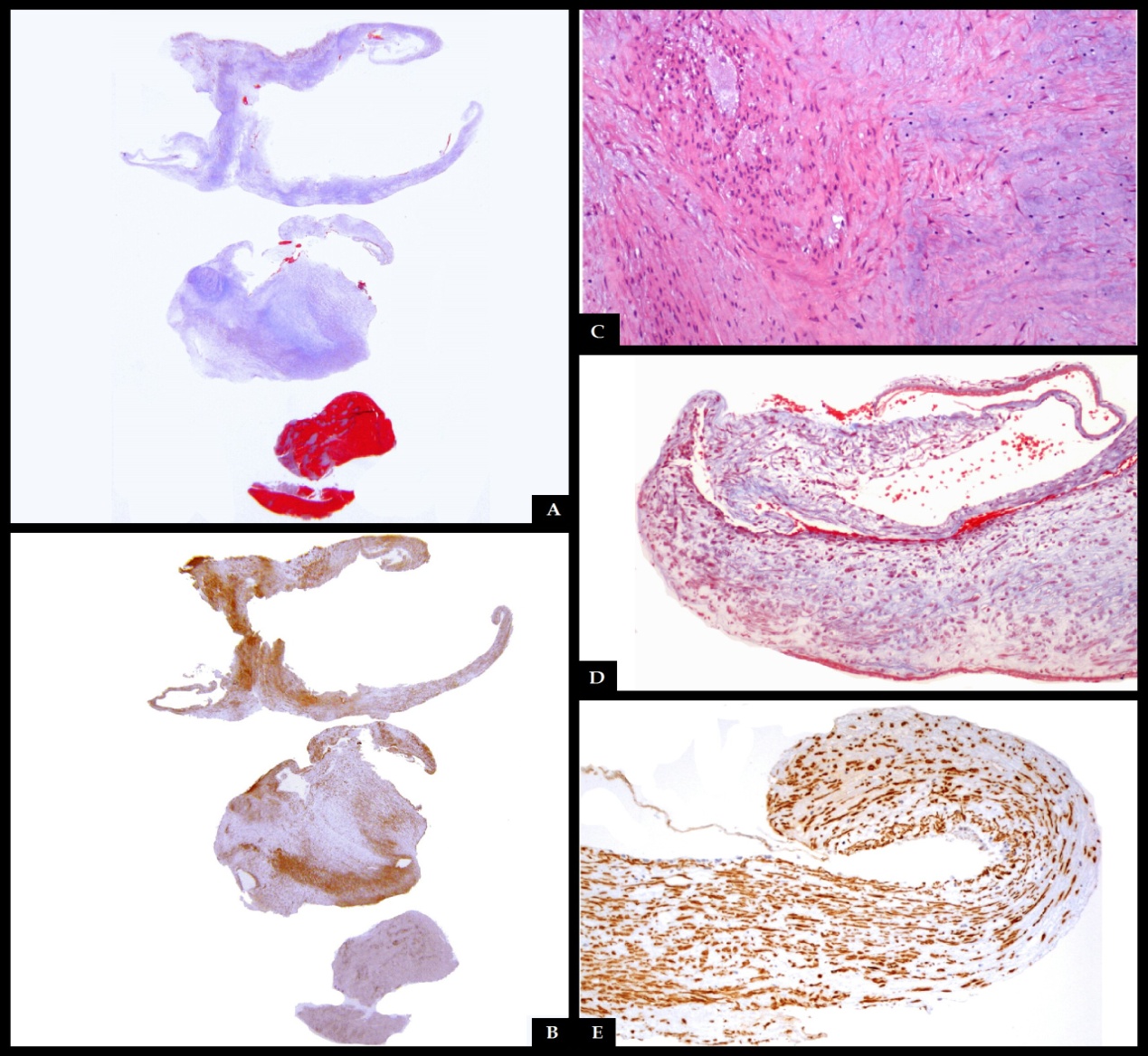
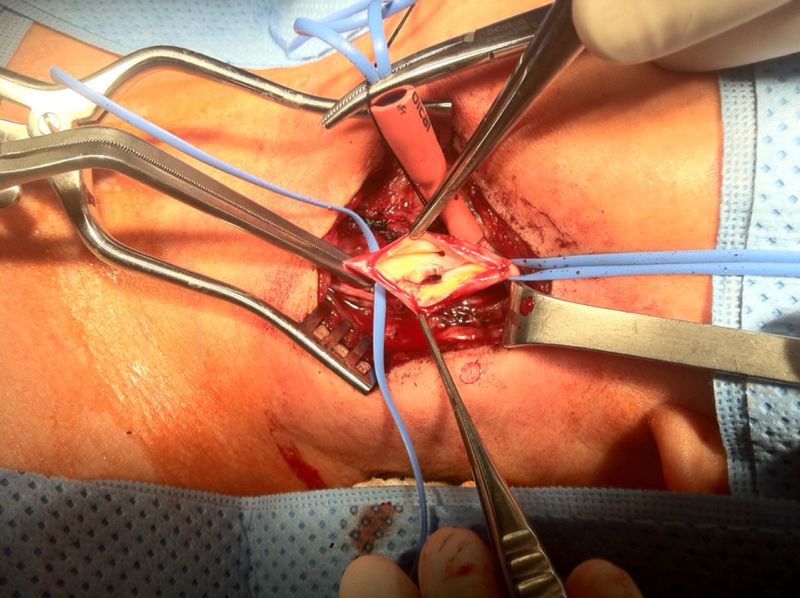 | Picture 3. Transoperative photograph during endarterectomy, showing intimal endoluminal protrusion of rugged tissue, with a clot inside its inner aspect |
3. Discussion
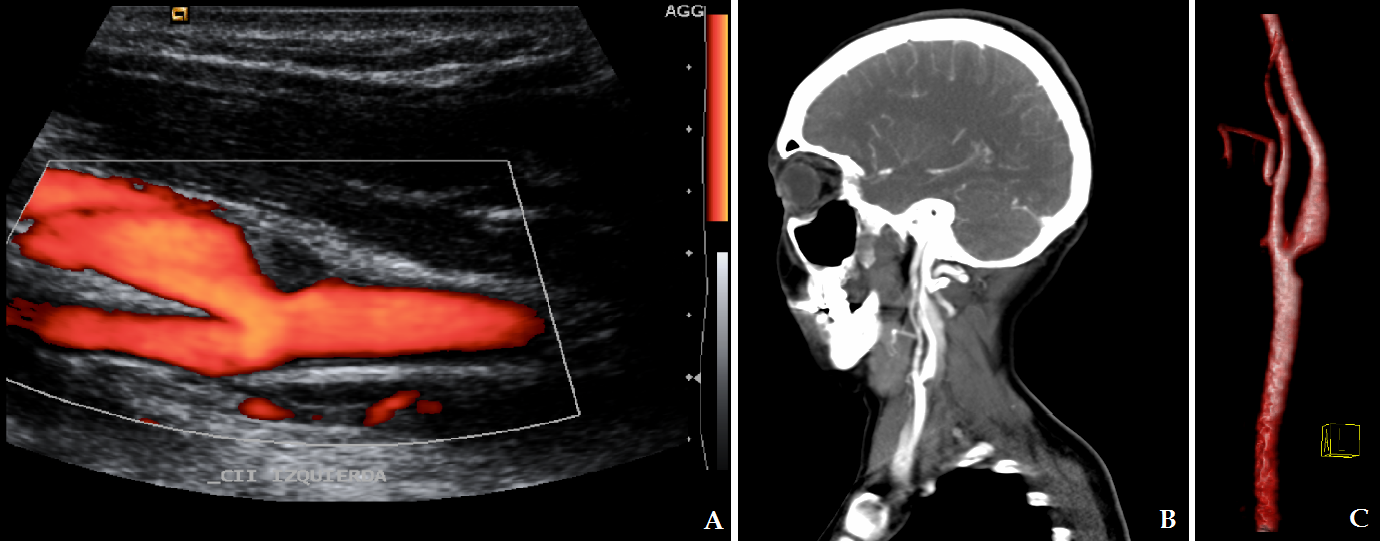
- FMD is recognized as a cause of renal artery stenosis, however it can affect almost all medium-and small-size arteries[1]. In cases of renal artery disease, arterial hypertension is the main clinical feature, and FMD is the leading cause of renovascular hypertension in young patients[5]. Most (95%) of all cases of cervical FMD are located in the internal carotid artery, most commonly in its middle or distal segments; lesions may be bilateral in 60 to 85% of cases. Involvement of vertebral arteries is less frequent, and generally coexists with carotid lesions[14]. Furthermore, 50% of patients with cervical arterial lesions also have renal artery disease[4, 9]. Some genetic causes, such as alpha-1 antitrypsin deficiency, as well as hormonal factors, certain toxins or drugs, and the presence of collagen diseases, have been described in the pathogenesis of this disorder[3, 14]. Smoking contributes to the development of vascular lesions by not-well understood pathogenetic mechanisms. Smokers with FMD have a more severe course of the disease[2]. FMD is sporadically associated with pheochromocytoma, neurofibromatosis, Ehlers-Danlos syndrome type IV, Alport syndrome, cystic medial necrosis and aortic coarctation[1].Three histological variants of FMD have been described, depending on the arterial wall layer that is affected[7, 8]. FMD type 1, which affects the tunica media, accounts for 85% of cases, and corresponds to areas of thinned media alternating with fibromuscular ridges also containing collagen, which replace normal tissue[4, 14]. FMD Type 2, or intimal fibroplasia (10% of cases), presents with circumferential or eccentric deposition of collagen in the tunica intima and the internal elastic lamina may be fragmented or duplicated[4, 14]. FMD type 3, which accounts for less than 5% of cases, is also called periadventitial fibroplasia, whose characteristic are dense nodular deposits of collagen that replace the connective tissue of the adventitia[4, 14]. These three histologic types are not mutually exclusive and there may be more than one layer affected in the same artery[2]. Classically, contrast angiography is the method of choice to diagnose FMD, the classic image being that of “string of beads” pattern of the affected arteries, caused by the succession between areas of wall narrowing and hypertrophy[4]. This is the pathognomonic morphology of the FMD type 1[15, 16]. In cases of intimal fibroplasia, the observed pattern is a smooth and long narrowing of tubular appearance in the affected area, which can be unifocal or multifocal[3, 14]. There is a third, rare angiographic pattern, presenting with a diverticular image on one side of the artery, which may progress to frank aneurysm formation[3]. CT and MRA are noninvasive alternatives to contrast angiography to evaluate the intracranial vascular bed, with similar results[3]. Vascular lesions occurring in FMD include intracranial aneurysms in 21-51 % of cases, spontaneous vascular dissection in 20%, carotid-cavernous fistulae, and thrombus formation in areas of irregular flow and turbulence[1, 4, 16]. In our case, MRA showed no evidence of aneurysms in the intracranial vessels. Diagnosis is made by neuroimaging. CT and MRI reveal ischemic or hemorrhagic lesions caused by arterial lesions[3, 19]. In our case CT scan was normal, but MRI showed acute ischemic lesions in separate vascular territories, suggesting an embolic cause of the stroke. Carotid Doppler ultrasonography is useful for demonstrating the thickening of the artery wall, and intraluminal thrombosis, but its usefulness is limited by the fact that most carotid malformations are found in the distal segments of the internal carotid artery, which are not consistently examined with this technique[3, 4]. Contrast arteriography has a high sensitivity and specificity for detecting imaging patterns characteristic of FMD, and is still considered the gold standard for diagnosis. However, it is an invasive procedure and has some risk of complications[3, 4]. Helical CT angiogram is similar to invasive angiography in its capacity to diagnose FMD. It has high sensitivity to detect the “string of beads” pattern of arterial fibrodysplasia, but is less effective in showing other patterns of the disease; therefore it is not the best technique for diagnosing cases of intimal or adventitial dysplasia[3, 19]. MRI angiography has lower sensitivity for cervical artery malformations than it has for intracranial vasculature, and certain artifacts such as patient’s movement or swallowing during the procedure may alter the generated image of neck vessels[4]. Full body angiography is recommended in patients in which carotid or vertebral FMD are found, since more than a half of these individuals also have affected renal arteries[5]. In the present report, ultrasonographic detection of the thrombosis was possible because it was located at the level of the common carotid bulb, an unusual place for cervical artery FMD, and CT angiogram was used for confirmation, without the need for contrast arteriography. Although rarely obtained, arterial wall biopsy gives the definitive diagnosis and histological type of FMD, mainly in cases with nonspecific data on imaging studies[3]. Treatment of FMD depends on the presence or absence of neurological symptoms and complications[3]. Patients with FMD who present with stroke or carotid dissection should be admitted to a stroke unit and anticoagulation treatment should be started[2, 3, 14]. Management of FMD includes balloon dilation of stenotic areas, resection-anastomosis, endarterectomy and stent angioplasty[14]. In cases of aneurysm rupture, clipping or coiling if the lesion is suggested[2]. Treatment should be individualized[10]. In our case, endarterectomy was performed to remove the thrombus and prevent new episodes of distal embolization, with the added utility that it allowed resection of the lesion for histopathological examination. After the intervention, antiplatelet agents are recommended to prevent recurrences [3, 9]. Recurrence of stroke is uncommon, even without long-term treatment, and a benign course of the disease can be expected after surgery[3, 10]. Asymptomatic patients must also have sequenced imaging studies[9]. Our case is unique for the histologic type of FMD (intimal fibroplasia), the location of the stenosis in the carotid bifurcation and the absence of a beaded pattern[20]. In the first confirmed case of carotid intimal FMD that presented with ischemic events, the lesion was located at the common carotid artery[21]. However, that case had a concentric tubular stenosis, not a unifocal protrusion as in our patient. There are some other cases of histologically confirmed carotid intimal fibroplasia with intraluminal protrusions, which present with thromboembolic events[20, 22]. The patient we are reporting is one of the first fifteen cases of carotid intimal FMD with a unifocal protrusion, a leaflet, that has been confirmed histologically, and perhaps the first one with these features with a proximal location in the common artery bifurcation rather than the distal internal carotid. It has been suggested that cases of carotid intimal FMD with protrusions into the vessel lumen have a higher thromboembolic risk than other more common types of fibrodysplastic malformations[20].
ACKNOWLEDGEMENTS
- We are grateful to Dr. Oscar Del Brutto for his feedback in this paper.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML