-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Sports Science
p-ISSN: 2169-8759 e-ISSN: 2169-8791
2020; 10(1): 15-22
doi:10.5923/j.sports.20201001.03

Impact of Transcranial Direct Stimulation Current on Sleep Depth among Student-Athletes
Jonathan Charest, Alexandre Marois, Celyne H. Bastien
École de psychologie, Université Laval, Québec, Canada
Correspondence to: Jonathan Charest, École de psychologie, Université Laval, Québec, Canada.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Introduction: Several studies have highlighted the glaring lack of sleep among student-athletes. Finding an intervention which will alleviate sleep difficulties is thus important. This study explores the impact of a transcranial direct stimulation current (tDCS) stimulation and the depth of sleep with the Odds Ratio Product (ORP) sleep scoring method among a sample of student-athletes with subclinical sleep issues. Methods: Thirty student-athletes (15 females, 15 males, age 21.1 ± 2.1 years) were recruited. All participants underwent a series of questions to rule out depressive and anxiety disorders or any specific tDCS exclusion criteria. All participants were advised to maintain their usual sleep schedule during the course of the study. Each participant underwent five nights of either tDCS or placebo stimulation. While subjective sleep was scored through a sleep diary, PSG was scored according to the ORP method. Results: Analyses indicated a marginal improvement after experimental tDCS on subjective total sleep time for both men and women and a general gender difference. However, tDCS did not appear to significantly impact the Odds Ratio Product computed from the polysomnographic data. Discussion: Individualized tDCS setting should be further explored because of the observed difference in total sleep time estimation between males and females.
Keywords: Sleep, Student-athletes, tDCS, ORP, Gender differences
Cite this paper: Jonathan Charest, Alexandre Marois, Celyne H. Bastien, Impact of Transcranial Direct Stimulation Current on Sleep Depth among Student-Athletes, International Journal of Sports Science, Vol. 10 No. 1, 2020, pp. 15-22. doi: 10.5923/j.sports.20201001.03.
1. Introduction
- The importance of sleep for optimal cognitive and athletic performances, specifically among athletes, has been highly reviewed [1,2]. While students do report a high prevalence of sleep difficulties, student-athletes are a subpopulation at greater risk of suffering from them due to numerous and widely different factors such as constant jetlag and travels [3], training load [4] and abnormally busy schedule including school and sometimes two workouts a day [5]. Furthermore, sleep plays a crucial role into muscle tissue and cognitive functions recovery [6,7]. Given the high physical and psychological demands that student-athletes face sleep represents a health pillar that should not be overseen.Polysomnography (PSG) is the cornerstone of investigation in sleep medicine regardless of patient age. Interpretation of sleep in scientific studies are mainly carried out with the scoring rules introduced by Rechtschaffen and Kales (R&K) more than 50 years ago [8]. In the late 90s, some members of the sleep medicine community complained about the lack of precision of the R&K rules, specifically regarding the microstructure of sleep [9]. Nowadays, it is widely recognized that R&K rules fail to distinguish EEG patterns between epochs of the same sleep stage [10]. An innovative approach to differentiate depth/quality of sleep between epochs of the same sleep stage has been developed by Dr. Younes and his team [11]. The Odd Ratio Product (ORP) is an index with a range between 0 and 2.5 predicting depth of sleep. The ORP range is separated into 3 states: 0 to 1.0 predicting sleep, range of 2.0 to 2.5 predicting wakefulness and a range between 1.0 to 2.0 predicting unstable sleep. Traditional sleep patterns from wakefulness to deep sleep, known as stage 3, were associated with a decreased ORP index by Younes and colleagues [11]. The association between ORP and arousability has also been shown by Younes and colleagues [11]. Hence, ORP can be a reliable alternative to measure sleep quality, especially with its ability to discriminate two epochs of a same sleep stage [11]. Given how recent ORP measures are, no study has hitherto used the ORP method to measure sleep within the student-athlete community. This new and single approach could lead to a better understanding of sleep microstructure regarding depth of sleep within a particular sleep stage. Given the few researches using PSG as a mean of sleep measurement with student-athletes, because it is time consuming and not readily available, ORP can be quite informative within this population. Moreover, one can even validate this technique of sleep deepness in a different population than the one studied by Younes and colleagues [11]. In addition, several gender differences in sleep pattern among student-athletes have been reported. In fact, according to Tsai and Li [12], female students go to bed earlier, wake up earlier, have longer sleep latency, more awakening and show a poorer sleep quality than males. For these multiple reasons, investigating sleep issues using the ORP method and assessing potential gender differences among a sample of student-athletes seems essential to improve knowledge on this population’s sleep habits and difficulties. Transcranial Direct Stimulation Current (tDCS) offers the possibility to non-invasively modulate cortical activity, which leads to opportunities for investigating the role of certain areas of the brain without undesirable effects [15], in a safe and painless way [16]. Currently, tDCS uses a constant, low intensity current which is delivered to the targeted cerebral region of interest through two electrodes placed over the head (one anode and one cathode) through which current flows [17]. tDCS bears an interesting potential in manipulating the total sleep time (TST) in humans. As shown by Frase and colleagues [13], TST was reduced with an anodal stimulation; however, cathodal stimulation failed to increase total sleep time due to a ceiling effect within their sample. A second study by Frase and colleagues [14] showed no tDCS effects between healthy participants and participants with insomnia disorder on sleep continuity and sleep architecture. Finally, a research conducted by Charest and colleagues [18] among student-athletes, also demonstrated the absence of impact of tDCS on sleep architecture even though a recent review raised its promising potential for alleviating insomnia symptoms [19]. These three studies were conducted using the traditional R&K rules. The lack of precision between epoch distinctions of R&K may however be the reason for the negative cathodal results in previously mentioned studies. Because the ORP index distinguishes two epochs of a same sleep stage, this precision may allow researchers to observe changes which could have been neglected under the R&K rules.Building upon Charest et al.'s work and data [18], the current study was designed to explore the impact of a tDCS stimulation on depth of sleep in student-athletes by using the ORP scoring method. To do so, student-athletes were separated in two groups, one group being exposed to the tDCS stimulation whereas the other group underwent a placebo stimulation. Furthermore, as gender differences in sleep quality, sleep architecture, sleep duration, sleep latency and sleep estimation have been previously reported throughout the general population and among student-athletes [12,20], secondary analyses were conducted to explore differences in objective and subjective sleep between men and women. Specifically, we tested the hypothesis that a frontal cathodal tDCS would decrease the ORP index in every category compared to a placebo among student-athletes.
2. Method
- ParticipantsThirty healthy student-athletes (15 females, 15 males) with a mean age of 21.1 years (SD = 2.1, range 18-25 years) were recruited with a non-probability sampling by reasoned choice to participate in a study at the research center of the Mental Health University Institute in Canada. One participant abandoned the protocol because of the inability to sleep with the polysomnography device. Missing data for seven participants (three from experimental group and four from the placebo group) were caused by movements of the sleep device during the night. Due to the inaccuracy of these nights, data were interpolated from the total. Participants provided written informed consent before the study. There was no monetary compensation offered to participants during this research. The study was conducted in accordance with the ethical committee of the research center of the Mental Health University Institute in Canada (project #2017-202).Inclusion and Exclusion CriteriaAll participants underwent a series of questions to rule out depressive (score > 13) and anxiety disorders (score > 19) or any specific tDCS exclusion criteria such as suffering from a concussion, wearing a pacemaker, pregnancy, repetitive migraine, wearing a metal implant and being epileptic. In addition, participants needed a PSQI score of > 5 and Insomnia Severity Index (ISI) score of > 7 to be included in the procedure. They were asked to maintain their usual sleep schedule to ensure ecological validity given that most sleep studies impose a specific bedtime and wake time for their participants, which does not always reflect their realities. All participants were asked not to consume any caffeine afternoon nor any alcohol.Apparatus and MaterialTranscranial direct current stimulation. tDCS was delivered by a battery-driven, micro-processor-controlled CE-certified constant current stimulator (NeuroConn GmbH, Illmenau Germany) comprising one targeted electrode (35 cm2, FPz) and a return electrode (35cm2, Pz) covered with a controlled saline water. Bifrontal stimulation was selected to target the “top-down” pathway of sleep regulation that has been targeted in previous research [13]. The target electrode used the standard size for an effective and safely stimulation [27]. For a powerful effect within the safety recommendations, a constant current of 2 mA was applied. A fade-in/fade-out design (30 seconds each) was used to decrease the potential skin sensation during the beginning and end of the stimulation [25]. The duration of the placebo procedure was 20 minutes with a 30 second fade-in at the beginning followed by a 30 second fade-out at the end without an active stimulation in between. This procedure has been reported to keep participants blinded for stimulation conditions [13,26]. At the end of the stimulation, participants were asked if they thought they received a real stimulation, a placebo stimulation or if they could not guess. Participants were asked not to randomly guess the procedure, if they were not sure, they had the choice of “undecided”. Only one participant was able to guess accurately the stimulation condition out of 30. Sleep Measures. The following questionnaires were administered to all participants and were filled out in person in a single sitting. All questionnaires asked participants to answer questions relating to their normal sleeping patterns over the previous month. Participants needed approximately 10 to 15 minutes to complete the questionnaires. These measures were garnered for potential exclusion criteria.The Pittsburgh Sleep Quality Index (PSQI). The Pittsburgh Sleep Quality Index (PSQI) is a self-rated 19-item instrument intended to assess sleep quality and sleep disturbance over a 1-month period in clinical and nonclinical populations [24]. Global scores range from 0 to 21 with higher scores indicating poorer overall sleep quality. The PSQI has been demonstrated to have good internal reliability, validity and is perhaps the most commonly used subjective sleep measure not only in the research literature, but also in the sleep community [24]. A recent investigation of PSQI psychometric values [25] indicated that the internal consistency was acceptable (Cronbach’s alpha = 0.75). The PSQI test-retest reliability is .87 within patient who scores 5 < [23]. A PSQI global score > 5 resulted in a sensitivity of 98.7 and specificity of 84.4 as a marker for sleep disturbances [26].The Insomnia Severity Index (ISI). The Insomnia Severity Index (ISI) is a self-reported, 7-item questionnaire designed to assess the subjective nature, severity and impact of symptoms associated with insomnia [27]. Each element is evaluated on a Likert scale between 0 (none) and 4 (extremely) for a total score of 0 to 28. An ISI score ≤ 7 indicates a clinically significant absence of insomnia, while an ISI score ≥ 15 indicates a clinical diagnosis of insomnia of moderate severity. The ISI was validated with a sample of 145 patients aged 41.4 years on average, consulting in a clinic specializing in sleep disorders related to insomnia. The test-retest reliability of the ISI is acceptable (r = 0.65). The average Cronbach alpha coefficient (α = 0.88) and the corrected item-total correlation average (r = 0.62) also reflect the high internal consistency of the ISI. The convergent validity obtained between the ISI and the sleep diary varies between r = .48 and r = .59.Other measures. The following questionnaires were administered at the beginning of the study to screen for depressive and anxious traits. These measures were also garnered for exclusion criteria and to screen for potential covariables.The Beck Depression Inventory (BDI). The Beck Depression Inventory (BDI) is a 21-question multiple-choice questionnaire used to measure the severity of symptoms of clinical depression [28]. A scale from 0 (no symptoms) to 3 (intense symptoms) is used for this test. The test–retest procedure indicated adequate stability over a 4 months period and has a reliability coefficient of 0.93 [28]. A total score of 0–13 is considered minimal range, 14–19 is mild, 20–28 is moderate, and 29–63 is severe [28]. The Beck Anxiety Inventory (BAI). Beck's Anxiety Inventory (BAI) consists of 21 symptoms of anxiety [29]. The respondent indicates, on a scale of 0 meaning "not at all" to 3 meaning "a lot", to what level each symptom affected him during the last week. The test–retest procedure indicated adequate stability over a 4-week period and has a reliability coefficient of 0.826 [29]. Total score of 0–9 is considered normal to minimal anxiety, 0–18 is mild to moderate anxiety, 19–29 is moderate to severe anxiety, and 30–63 represents severe anxiety [30]. Sleep RecordingPolysomnography was recorded once participants were ready to go to bed, but not later than midnight. The Prodigy Sleep Monitor/Michele Sleep Scoring System was the device used during the course of this study. This device is manufactured by Younes Medical Technologies, an ISO 13485 certified company. Dual frontal EEG, dual EOG, intercostal and chin EMGs, nasal cannula ECG, head and body position electrodes were used for sleep recording. All polysomnographic recordings were scored by an automatic sleep scoring system (Michele) and revised by a trained and experienced rater that was blinded to the study. The following polysomnographic parameters of sleep continuity and architecture were assessed: ORP 0-1, ORP 1-2, ORP 2-2.5, ORP ASO, ORP Sleep and ORP TST. Average ORPs were calculated in each sleep parameter. Procedure and Study DesignBefore completing any step of the study, individuals gave their informed consent to participate in the study. Interested participants completed sleep questionnaires, depressive and anxiety inventories before baseline. All participants underwent a within-subject, repeated-measures protocol across five nights at their own house. Thirty individuals were enrolled and no attrition was observed throughout the course of study. Participants were double blinded randomized into two different groups: placebo and experimental. The placebo group was represented by 15 student-athletes (8 females) and the experimental group comprised the other 15 student-athletes (7 females). One adaption night (Night 1) was followed by two baseline nights of polysomnography at home (Night 2-Night 3). During the adaption night, participants were monitored for sleep apnea using a nasal thermistor and the Apnea-Hypopnea Index (AHI). Five days following Night 3, two tDCS or two placebo tDCS nights were performed at home (Night 4-Night 5). tDCS was applied by the experimenter 90 minutes before bedtime for twenty consecutives minutes. Figure 1 depicts the design of the study.
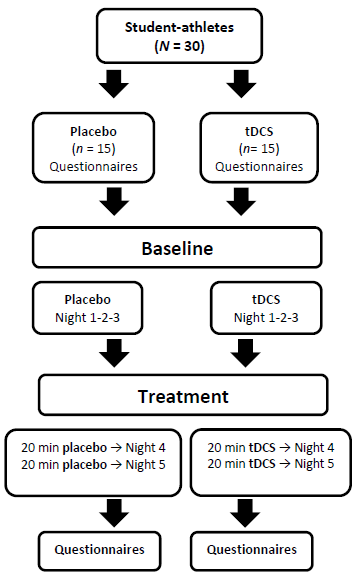 | Figure 1. Study timeline and protocol (see also [18]) |
3. Results
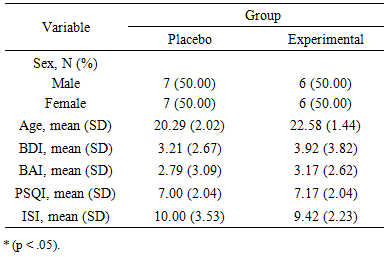
- Characteristics of the sample are presented in Table 1. Both groups did not differ regarding the depressive affects, t(24) = 0.05, p = .961, nor the anxiety level, t(24) = 0.64, p = .286, as measured by the BDI and BAI, respectively. All participants were below the clinical threshold for either depression (3.21 ± 2.67; 3.92 ± 3.82) or anxiety (2.79 ± 3.09; 3.17 ± 2.62) before the study. All participants reached the clinical threshold for either PSQI (7.00 ± 2.04; 7.17 ± 2.04) and ISI (10.00 ± 3.53; 9.42 ± 2.23) before the study.
|
|
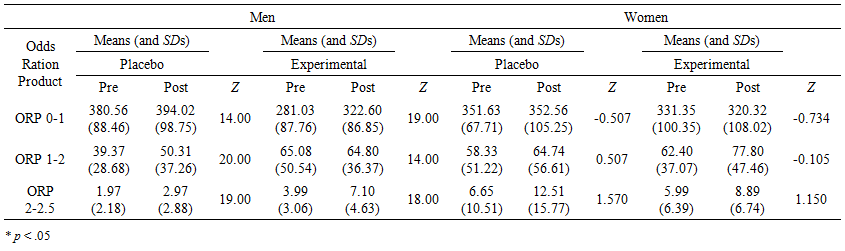 | Table 3. Means, Standard Deviations and Group Wilcoxon signed-rank test results for each ORP variable of sleep for both placebo and experimental groups at pre- and post-stimulation by gender |
|
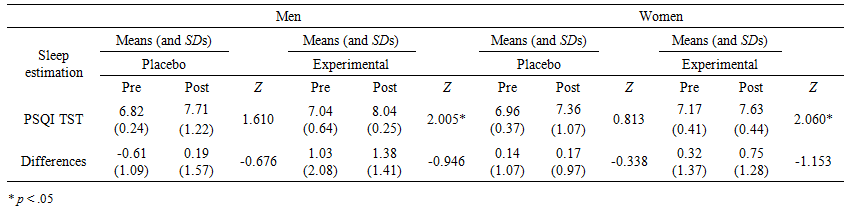 | Table 5. Means, Standard Deviations and Group Wilcoxon signed-rank test results for each sleep estimation variable for both placebo and experimental groups at pre- and post-stimulation by gender |
4. Discussion
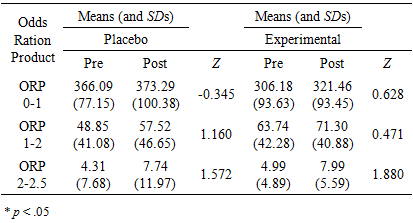
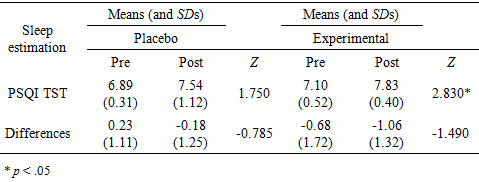
- Sleep is a predominant issue that is overseen among student-athletes. This is the first investigation that explores the impact of tDCS on the depth of sleep with a new index: the odds ratio product. Following up Charest et al.’s [18] previous work, sleep was objectively assessed within five nights of polysomnography using the ORP method. Additionally, subjective sleep estimation was assessed with PSQI and men-women differences in sleep were investigated. Results showed that neither the placebo nor the experimental group significantly increased or decreased their ORP, regardless of gender. Additionally, contrary to our hypothesis, the experimental group did not decrease their ORP index in any of the objective sleep categories. Nevertheless, participants from the experimental group subjectively increased their total sleep time. Both genders had similar results regarding the subjective total sleep time assessed by the PSQI. Furthermore, following tDCS, our results indicated that men significantly increased their subjective total sleep time compared to women. These results bear multiple interrogations. First, the inverse tendency of tDCS stimulation on objective total sleep time and subjective sleep time among women. Second, the difference tendency between men and women after a tDCS stimulation. Last, even if this was not statistically significant, there was an increased ORP index 2-2.5 tendency in both groups, placebo and experimental, regardless of gender.Gender DifferencesGender differences exist in sleep quality, sleep architecture, sleep duration and sleep latency throughout the general population and student-athletes [12,20]. Our results indicated that both genders, following a tDCS stimulation, estimated a longer sleep duration. However, there was an objective increase in ORP index 0-1 only in men contrary to women who exhibited a decrease in the same index. While men in the experimental group increased their ORP index 0-1 by 41.57 minutes on average, the women decreased the same index by 11.03 minutes on average. As it was shown by Younes and colleagues [11], the depth and quality of sleep is represented by the amount of time a sleeper spend in the lower ORP index (0-1). The increased ORP index 0-1 would suggest that men benefited more from an anodal tDCS stimulation on the prefrontal cortices than women. This gender difference could be explained by the fact that men have more cancellous parietal bone and women more dense parietal bone [31]. In fact, the skull is a high resistance and anatomically complex structure that has a major impact on the density of the current. Previous research have attributed gender differences in current stimulation to hormonal conditions and neuroplasticity differences [32]. However, Russell and colleagues [31] indicated that for the same current and electrode size, men received significantly more current than women and this could be due to the bone density differences. In addition, with, again the same current and electrode size, there were large differences in the amount of current that the participants received when comparisons were made between frontal and parietal sites. These differences were not considered in our procedure and could explain the difference between men in women in our experimental groups. Earlier studies have reported that the inconsistency in tDCS results may be attributed to individual differences in head anatomy [33]. With the inconsistency that is known when it comes to tDCS results, these differences call for an individualized protocol paired with the density of bones and intensity of the current accordingly with the gender of the participant.Sleep EstimationOur results indicate that regardless of gender, following a tDCS stimulation, both groups increased their total sleep time estimation by 49.20 minutes on average. As it was recently showed, positive total sleep time estimation is linked with better sleep quality and daytime functioning [34]. Men in the experimental group increased their perceived total sleep time by 60 minutes on average compared to 45.60 on average for women. After tDCS stimulation, both groups had a similar total sleep time according to polysomnography, but men tended to overestimate their sleep duration. This is in line with the literature. Results from Short and colleagues [35] indicated that men actigraphy showed poorer sleep compared to women but longer sleep duration on sleep diaries. This could be explained by the quantity of movements during sleep by men. It appears that men tend to move more than women during sleep, thus the inability of polysomnography to score adequately sleep when compared to sleep diaries [35]. According to multiple sleep researches, women have better polysomnographic sleep quality than men [36,37]. Despite numerous reports that women have better sleep quality, a paradox does exist. Women subjectively report more sleep problems such as difficulty falling asleep, staying asleep and long period of awakening during the night [38]. Our results indicate that women have a tendency to spend more time in both ORP indices (1-2 and 2-2.5) that represent shallow sleep compared to men. Even if their sleep duration is similar, this may be an indication of lower sleep quality. Thus, it may explain the lower subjective sleep assessment by women. Again, these results underline the fundamental need of individualized approach to sleep in both genders. An individualized tDCS approach which would consider the anatomy of the participant’s head (to minimize interference with the current density) should always be taken into consideration. This could address the inconsistency of tDCS results throughout the literature. In addition, since the sleep habits of men and women are different, it is important for both genders to be evaluated accordingly in order to optimize the outcomes.
5. Conclusions
- Our research has demonstrated that tDCS may have a positive impact on subjective sleep. This is also the first research conducted among student-athletes which uses the novel method of Odds Ration Product, that is a sleep scoring system measuring continuously quality and depth of sleep. As it was observed in our study, tDCS could lead toward an increased total sleep time estimation in men, which is ultimately link to better daytime functioning [34]. Additionally, given that tDCS is a non-pharmacological approach, its enhancement would be realized without any sleep medications and their known side effects. However, this research acknowledges the different impacts of tDCS between men and women. Given the variability in tDCS results through the literature [19] and our study, it would be warranted to conduct more research to develop robust finding before considering the implementation of tDCS as a viable treatment.Additionally, despite its innovative perspective, our study relied on a small sample size which may have increased alpha errors given the number of tests. Nevertheless, with the few significant results, this is most unlikely. These findings are highly relevant given that student-athletes remain extremely vulnerable to poor sleep quality and quantity, and that there is a lack of available non-pharmacological interventions for them. Future research should replicate the present protocol with a higher number of participants in order to better investigate the impact of tDCS on ORP and gender differences.
ACKNOWLEDGEMENTS
- We thank the athletes for participating in the study. We also thank all the persons involved in this study at Cerebra Health.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML