-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Sports Science
p-ISSN: 2169-8759 e-ISSN: 2169-8791
2019; 9(4): 69-85
doi:10.5923/j.sports.20190904.01

Systematic Literature Review: The Effect of Dairy Milk on Markers of Recovery Optimisation in Response to Endurance Exercise
Isabella Russo1, Vera Camões-Costa1, Stephanie K. Gaskell1, Judi Porter1, 2, Louise M. Burke3, 4, Ricardo J. S. Costa1
1Department of Nutrition Dietetics & Food, Monash University, Notting Hill, Australia
2Allied Health Clinical Research Office, Eastern Health, Box Hill, Australia
3Australian Institute of Sport, Canberra, Australia
4Mary MacKillop Institute for Health Research, Australian Catholic University, Melbourne, Australia
Correspondence to: Ricardo J. S. Costa, Department of Nutrition Dietetics & Food, Monash University, Notting Hill, Australia.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The food and fluid provided in the acute post-exercise period plays an essential role in endurance exercise recovery and adaptation. The current systematic literature review (SLR) aimed to identify and synthesize research that investigated the effect of dairy milk beverages in comparison to alternative post-exercise beverages on markers of ‘exercise recovery optimisation’, which may influence subsequent endurance exercise performance. Seventeen papers met the inclusion criteria. Quality assessment was undertaken using the Cochrane Collaboration’s tool for assessing risk of bias. Intervention beverages included fresh dairy milk (n= 3), chocolate flavoured dairy milk (n= 6), dairy milk-based sports beverages (n= 4), or a combination of the aforementioned beverages (n= 4). Results indicate dairy milk enhanced muscle protein synthesis (i.e., mixed fractional synthetic rate: 0.11%/h dairy milk vs. 0.08%/h control), and elicited similar rates of muscle glycogen resynthesis (5.9 mmol/kgWW/h) compared to a carbohydrate replacement beverage (7.6 mmol/kgWW/h). Seven studies investigated the effect of dairy milk beverages on hydration status, three of which found no differences in restoring net fluid balance after consumption of a dairy milk or dairy milk-based beverage compared to a carbohydrate-electrolyte beverage and (or) water, when consumed ad libitum. The remaining four studies observed a greater net fluid balance after consumption of a dairy milk or dairy milk-based beverage compared to an isovolumetric dose of a carbohydrate-electrolyte beverage and (or) water. To date, no study has investigated the effect of dairy milk consumption after endurance exercise on markers of immune competency or gastrointestinal status. Five studies observed enhanced time-trial or time-to-exhaustion performance (7.4% to 52.4%) with a dairy milk beverage compared to an isocaloric beverage, while another study found no differences. It is concluded that dairy milk may provide either comparable or superior recovery nutrition qualities with regards to muscle protein synthesis, glycogen replenishment, rehydration, and subsequent endurance exercise performance, when compared to non-nutritive, carbohydrate replacement, and (or) carbohydrate-electrolyte alternatives.
Keywords: Muscle glycogen, Muscle protein synthesis, Rehydration, Immune function, Gastrointestinal
Cite this paper: Isabella Russo, Vera Camões-Costa, Stephanie K. Gaskell, Judi Porter, Louise M. Burke, Ricardo J. S. Costa, Systematic Literature Review: The Effect of Dairy Milk on Markers of Recovery Optimisation in Response to Endurance Exercise, International Journal of Sports Science, Vol. 9 No. 4, 2019, pp. 69-85. doi: 10.5923/j.sports.20190904.01.
Article Outline
1. Introduction
- It is well established that the food and fluid consumed in the acute period after endurance exercise plays an essential role in metabolic and physiological recovery, and biological adaptation processes to the respective exertional stress. Replacement of carbohydrate due to muscle glycogen depletion (e.g., oxidative phosphorylation of endogenous carbohydrate) and water due to exercise-associated body water losses (e.g., sweat, respiration, and obligatory urine losses), in the initial hours following exertional stress is required to prevent decrements in performance during subsequent training or competitive events [1-3]. Moreover, promotion of adaptive responses to endurance training, including accretion of mitochondrial and myofibrillar muscle proteins can be modulated through post-exercise nutritional intake, most appreciably due to the quantity and quality of protein intake [4,5]. There has been a considerable amount of research investigating the effects of nutritional strategies on recovery from prolonged endurance exercise, which has led to the development of nutrition guidelines and recommendations for the ideal quantity and (or) quality of carbohydrate, protein, and water to optimally replenish muscle glycogen stores, maximally stimulate net positive muscle protein synthesis, and restore hydration status, respectively [1,6]. However, the majority of exercise recovery nutrition research has neglected immunocompetency in their recovery assessment, and also neglected the integrity and function of the gastrointestinal tract, with feeding tolerance and gastrointestinal symptoms (GIS) being measured occasionally in an inconsistent manner and (or) briefly mentioned [7]. Considering immunocompetency and a competent gastrointestinal tract are essential pre-requisites for optimal nutrient intake and exercise recovery [8-11], the current research may have underestimated the potential for further muscle glycogen replenishment, muscle protein synthesis, and rehydration, leading to suboptimal exercise recovery nutrition guidelines and recommendations.There is increasing evidence to suggest that dairy milk, in particular chocolate flavoured dairy milk (CM), has the potential to provide a ‘gold standard’ recovery formulation, since it has similar nutritional properties to current exercise recovery nutrition guidelines and recommendations [12-14]. Partly skimmed (2% fat) CM contains carbohydrate and protein (casein and whey) in an approximate ratio of 3:1. Post-exercise consumption of carbohydrate and protein in this ratio (i.e., 0.8-1.2 g/kg and 0.3-0.4 g/kg, respectively) has been reported to enhance muscle protein synthesis, and have comparable effects on muscle glycogen resynthesis, and subsequent endurance exercise performance, compared to a beverage containing an isocaloric dose of carbohydrate [15-17]. Moreover, the natural mineral content of CM (i.e, sodium and potassium) is comparable to the concentration found in carbohydrate-electrolyte beverages (CEB) and may aid water retention in plasma and (or) intracellular compartments [18,19]. Considering this nutritional profile, the effectiveness of dairy milk as an exercise recovery beverage has been reviewed extensively using narrative methodologies, with a primary focus on anabolic processes (e.g. muscle glycogen and protein synthesis) and performance [20-24]. These reviews highlight the potential for dairy milk beverages to support recovery processes in both endurance and resistance exercise. A recent literature review examined the effectiveness of dairy milk recovery beverages on hydration status and subjective rating of thirst [22]. While there is some evidence to suggest that milk may be a more efficient hydration beverage than water or carbohydrate-electrolyte beverage [19], the search strategy implemented by this review only yielded four studies, and as a result, the authors concluded there was insufficient evidence to differentiate the effectiveness of dairy milk from other recovery beverages for rehydration. To date, only one systematic literature review (SLR) has examining the effects of CM on subsequent exercise performance, in adjunct with associated physiological strain and (or) recovery markers [23]. The meta-analysis within found that consuming CM after a preload exercise bout increases time to exhaustion (TTE) in a subsequent performance test, when compared to a placebo. However, this SLR did not evaluate the effects of dairy milk recovery beverages on muscle glycogen resynthesis, rehydration, immune function, or gastrointestinal status. In summary, the existing literature lacks a complete and systematic review of the effects of dairy milk and (or) dairy milk-based beverages on ‘exercise recovery optimisation’ (i.e., to maximise desired and minimise detrimental outcomes within the complex and interrelated physiological and metabolical homeostatic systems) that may be targeted by nutrition interventions. Therefore, the aim of the current SLR was to systematically identify and synthesize research that investigated the effect of dairy milk in comparison to alternative post-exercise beverages on markers of ‘exercise recovery optimisation’ (i.e., muscle glycogen resynthesis, muscle protein synthesis, hydration status, immunocompetency, and gastrointestinal status) and subsequent endurance exercise.
2. Methods
- This review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement [25]. The review protocol was registered (http://www.crd.york.ac.uk/PROSPERO) with PROSPERO, registration number CRD42017083594.
2.1. Search Strategy
- A three-step search strategy was developed with the assistance of an academic librarian. This search of published English-language studies was implemented across six electronic databases from inception until June 2019: Ovid MEDLINE, EMBASE, Cinahl, SportsDISCUS, Web of Science, and Scopus. The reference lists of all identified studies were searched to identify any additional studies for inclusion. The keywords applied in the literature search are shown in Table 1.
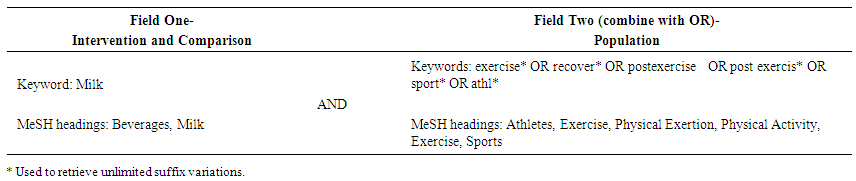 | Table 1. Search strategy for the systematic literature review on the effect of dairy milk in comparison to alternative post-exercise beverages on aspects of endurance exercise recovery |
2.2. Eligibility Criteria
- In order to obtain the level of methodological detail required, only laboratory controlled studies and field studies were considered for review. The Participant Intervention Comparator Outcomes Study design format (PICOS) was used to determine whether studies were eligible for inclusion (Table 2).
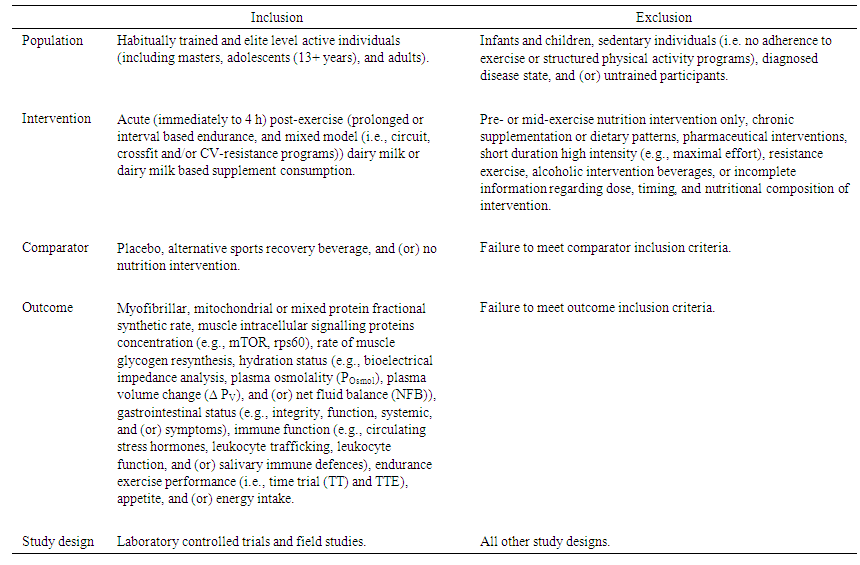 | Table 2. PICOS statement outlining inclusion and exclusion criteria |
2.3. Study Selection
- The database search was imported into Endnote and duplicates were manually removed. The searches were then imported into an online software program (Covidence) for management of studies and synthesis of evidence. Two reviewers (IR and VC) worked independently and in duplicate to assess papers against the PICOS. A third reviewer (RC) resolved conflicts where these arose. Full texts were also assessed against the PICOS statement by reviewers (IR and VC) independently and in duplicate, with consensus reached by a third reviewer (RC).
2.4. Data Extraction and Synthesis
- Data were independently extracted in duplicate (IR and VC) from each included study into a standardised table developed for reporting this review. Extracted data related to study characteristics, exercise stress, nutrition intervention, primary and secondary outcomes. Results were analysed descriptively due to the heterogeneity of study designs and reported outcomes; and therefore, extracted data were ineligible for further analysis (i.e., meta-analysis).
2.5. Risk of Bias Assessment
- Risk of bias assessment was performed using the Cochrane ‘risk of bias’ assessment tool [26]. Two reviewers independently (IR and SG) and in duplicate conducted the assessments by referring to the criteria for judging risk of bias in the assessment tool.
3. Results
3.1. Search Results
- The search strategy yielded 1,940 non-duplicate studies, including an additional study retrieved through screening of reference lists. Upon title and abstract screening, 1899 were excluded. The full texts of 41 studies were reviewed. Reasons for exclusion following full text screening include ineligible intervention design (e.g., resistance exercise intervention (n= 5), extended training program (n= 4), and non-milk intervention (n= 3)), abstract text or thesis only (n= 10), ineligible method for measuring outcomes (i.e., whole body protein turnover estimated through nitrogen excretion) (n= 1) or ineligible outcomes (i.e., blood glucose, insulin, cortisol and IL-6 concentration only) (n= 1). A total of 17 randomised crossover trials were included for review (Figure 1).
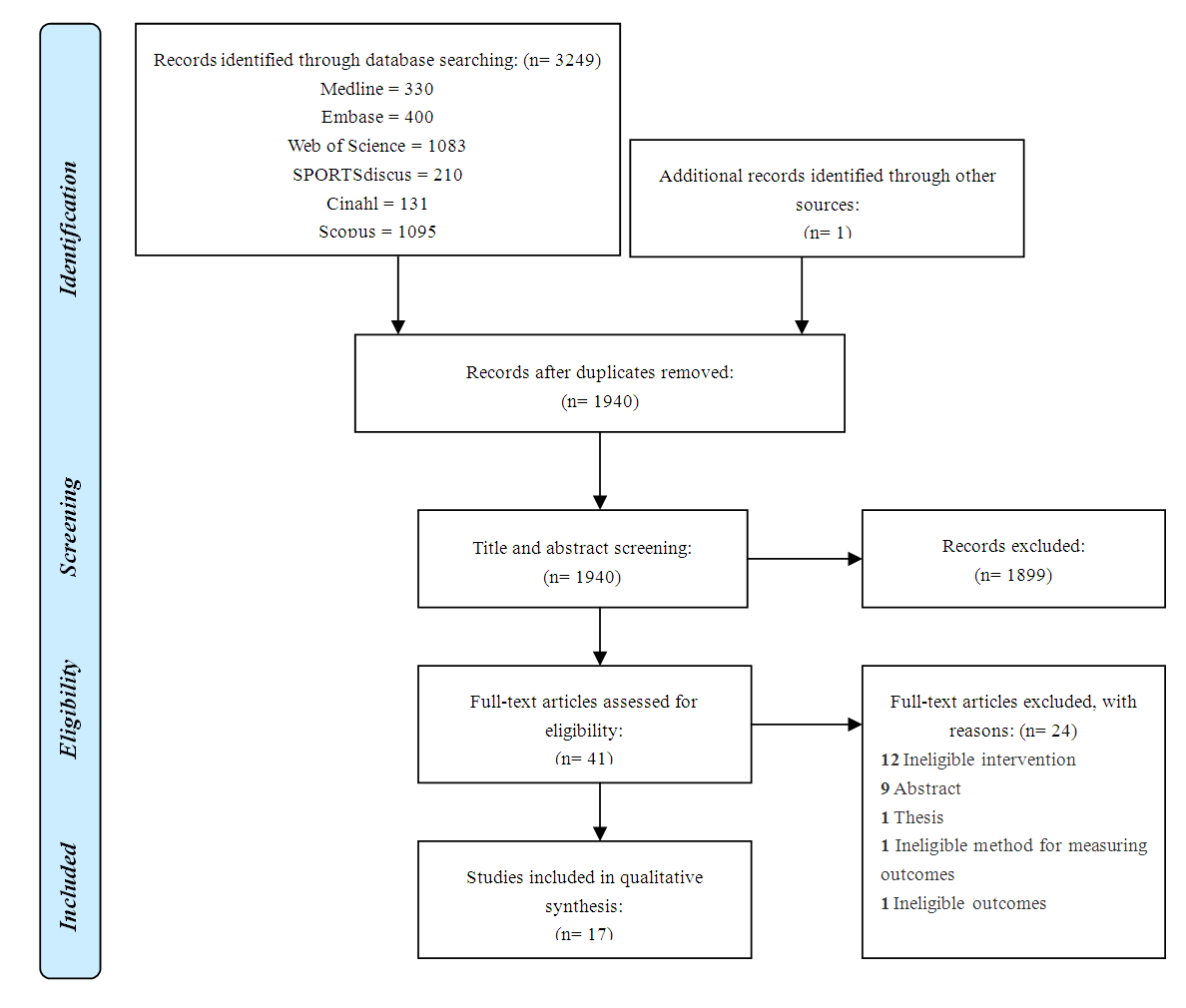 | Figure 1. PRISMA diagram, indicating the systematic review process, and subsequent inclusion and exclusion of respective research papers |
3.2. Study Characteristics
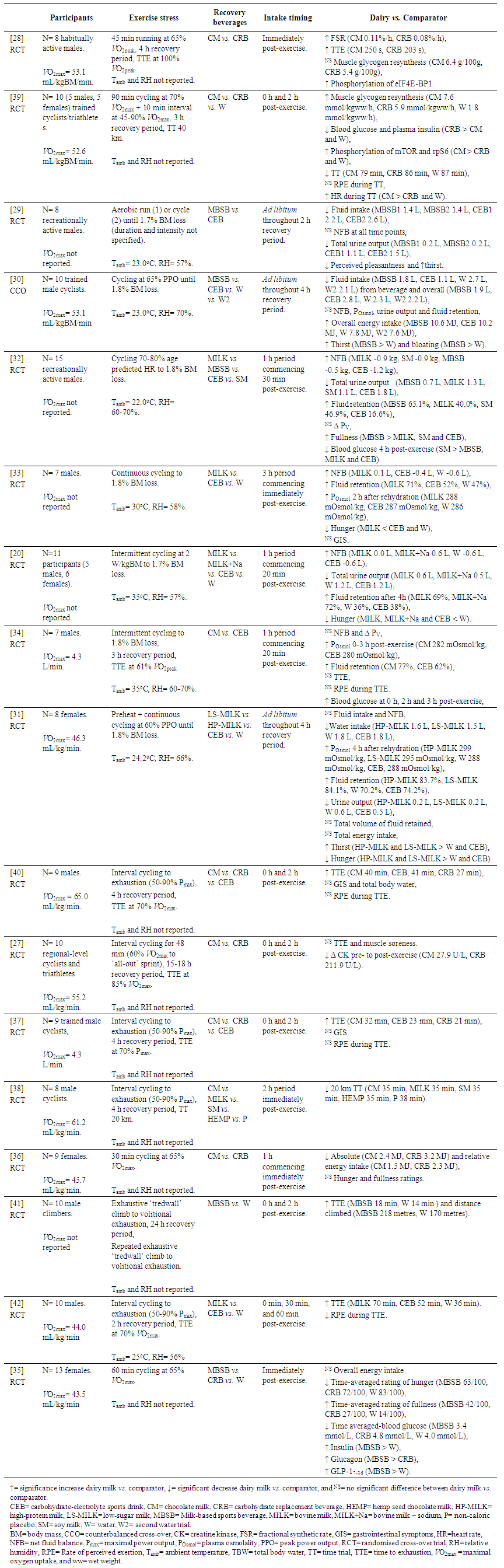
- The majority of participants in the included studies were male (74% male, excluding Pritchett et al. [27] where gender was not specified) with the mean age of participants ranging from 19 to 32 years. Fourteen of the included studies utilised cycle ergometry as the exercise modality, with the remaining studies utilising a treadwall (n= 1), treadmill (n= 1) or participants choice for either treadmill running or ergometer cycling (n= 1). The intensity, duration, and ambient conditions of the exercise stress is summarised in Table 2. Intervention beverages included fresh dairy milk (n= 3), CM (n= 6) and dairy milk-based sports beverages (MBSB) (n= 4). The remaining four studies compared a combination of the dairy milk and dairy milk-based beverages. Given the heterogeneous study designs, nutrition interventions and outcome measures, these results have been summarised descriptively in Table 3.
3.3. Effect of Dairy Milk on Muscle Glycogen Resynthesis and Muscle Protein Synthesis
- Two studies assessed the effect of dairy milk exercise recovery beverages on muscle protein synthesis and glycogen resynthesis. Lunn et al. [28] demonstrated that consumption of CM (0.2 g protein (PRO)/kg/h) after 45 min treadmill running at 65% V̇O2max stimulated mixed muscle protein synthesis greater than an energy-matched non-nitrogenous CEB (0.11%/h CM vs. 0.08%/h CEB; p <0.05). In this study, the CM and CEB provided 0.8 g carbohydrate (CHO)/kg/h and 1.0 g CHO/kg/h, respectively. There was no significant difference in the rate of muscle glycogen resynthesis between trials. It was also found that CM containing 0.6 g CHO/kg/h + 0.2 g PRO/kg/h and CRB containing 0.8 g CHO/kg/h elicited similar rates of muscle glycogen resynthesis [39]. Phosphorylation of the signalling proteins mammalian target of rapamycin (mTOR), eukaryotic initiation factor 4E-binding protein 1 (eIF4E-BP1) and ribosomal protein S6 (rpS6) was increased during the dairy milk trial, which is indicative of up-regulation of the mTOR muscle protein synthetic pathway [28,39].Four studies investigated the effects of dairy milk and (or) dairy milk-based beverages on blood glucose response, compared to a carbohydrate replacement beverage, soymilk, and (or) a non-caloric placebo. The blood glucose response during the recovery period for each study was variable, with no consistent pattern in terms of beverage type, energy or carbohydrate content (Table 2).
3.4. Effect of Dairy Milk on Hydration Status
- The search strategy retrieved seven studies that examined the effects of dairy milk or MBSB on hydration status. Of these, three studies investigated the effects of ad libitum intake of a MBSB [29,30], or a low sugar (LS-MILK) and a high protein (HP-MILK) dairy milk beverage [31], compared to water and (or) CEB. Two studies found intake of the MBSB was significantly less than water and (or) CEB. Another study found no significant differences in the volume of exercise recovery beverages consumed; however, significantly less water was consumed during both dairy milk trials, due to the higher energy density of the milk beverages (p≤0.022) [31]. Moreover, it was found that total water intake during the exercise recovery period (i.e., ab libitum) was significantly less during the dairy milk trials compared to CEB and water [30,31].Despite differences in total fluid intake, none of the aforementioned studies observed differences in net fluid balance (NFB) at any time point throughout the recovery time period measured. Offsets in NFB were likely due to corresponding increases in urine output with greater water intake, although these differences were not always significant [30]. McCartney et al. [31], however, observed a significantly higher POsmol 4 h after rehydration with the dairy milk trials (HP-MILK and LS-MILK > water, and HP-MILK > CEB), possibly associated with the nutrient density and bioavailability of dairy milk (Table 3). Interestingly, a higher percentage of fluid retention with LS-MILK compared with water was also observed, but there were no significant differences when corrected to total volume of fluid retained.Four studies provided participants with isovolumetric doses of exercise recovery beverages during each trial [32-34]. These studies observed a significantly greater positive NFB after consumption of dairy milk or MBSB, compared to water or CEB. With the exception of Watson et al. [34], the percentage of fluid retained was also significantly greater during the milk trials. Similar to the observations by McCartney et al. [31], two additional studies found that POsmol was significantly higher 2 h after the onset of rehydration during the dairy milk trials compared to CEB and (or) water [33,34].
3.5. Effect of Dairy Milk on Immune Function
- The search strategy did not yield any studies investigating the effect of post-exercise consumption of dairy milk on immune indices, such as total and differencial leukocyte trafficking, immune cell function (i.e., in-vitro or in-vivo), and (or) inflammatory cytokine responses.
3.6. Effect of Dairy Milk on Gastrointestinal Status and Intake Tolerance
- None of the reviewed studies observed any differences between trials in gastrointestinal discomfort per se. To date, no study has comprehensively assessed gastrointestinal discomfort and (or) GIS during the exercise recovery period in response to recovery beverage consumption using a validated and reliability verified GIS assessment tool [7].Nine studies reported on appetite and thirst throughout the exercise recovery period, five of which observed significantly lower levels of hunger and (or) increased levels of fullness and bloating after consumption of dairy milk compared to other trials [32-35], with another study reporting a trend towards significance [37]. Three studies found no differences in hunger between trials. However, two of these studies provided ad libitum food to participants during the exercise recovery period [29,30,36].Three studies investigated the effects of dairy milk or dairy milk-based exercise recovery beverages on ad libitum energy intake. All studies found energy intake from food during the exercise recovery period was significantly less during the dairy milk and (or) MBSB trial when compared with water [30,35], CRB [36], and CEB trials [30]. However, only two studies observed significantly lower overall energy intake during the recovery time period compared to the water [30] and CRB [36] trials.
3.7. Effect of Dairy Milk on Endurance Exercise Performance
- Nine studies investigated the effect of dairy milk and (or) MBSB on subsequent endurance exercise performance or capacity, which included a 20 km and 40 km cycling ergometer TT [38,39] or TTE at a given power output or speed [27,28,34,37,40-42], respectively. The majority of these studies conducted the performance test at the end of the 2-4 h recovery period, whereas two studies conducted the test the following day [27,41]. Where water or a non-caloric placebo was provided as a comparator, participants demonstrated greater endurance exercise performance (i.e., increased TTE or reduced TT) after consuming dairy milk as an exercise recovery beverage (8.6% to 93.3% greater performance) [38,39,41,42]. Six studies provided an isocaloric CRB or CEB as a comparator [27,28,37,39,40,42], five of which observed greater performance during the dairy milk trials (7.4% to 52.4% greater performance) [28,37,39,40,42]. Moreover, three studies compared the effects of dairy milk beverages to a lower-energy CEB. One study observed a greater TTE compared with the CEB [37], the remaining two studies found no significant differences [34,40].
3.8. Risk of Bias Assessment
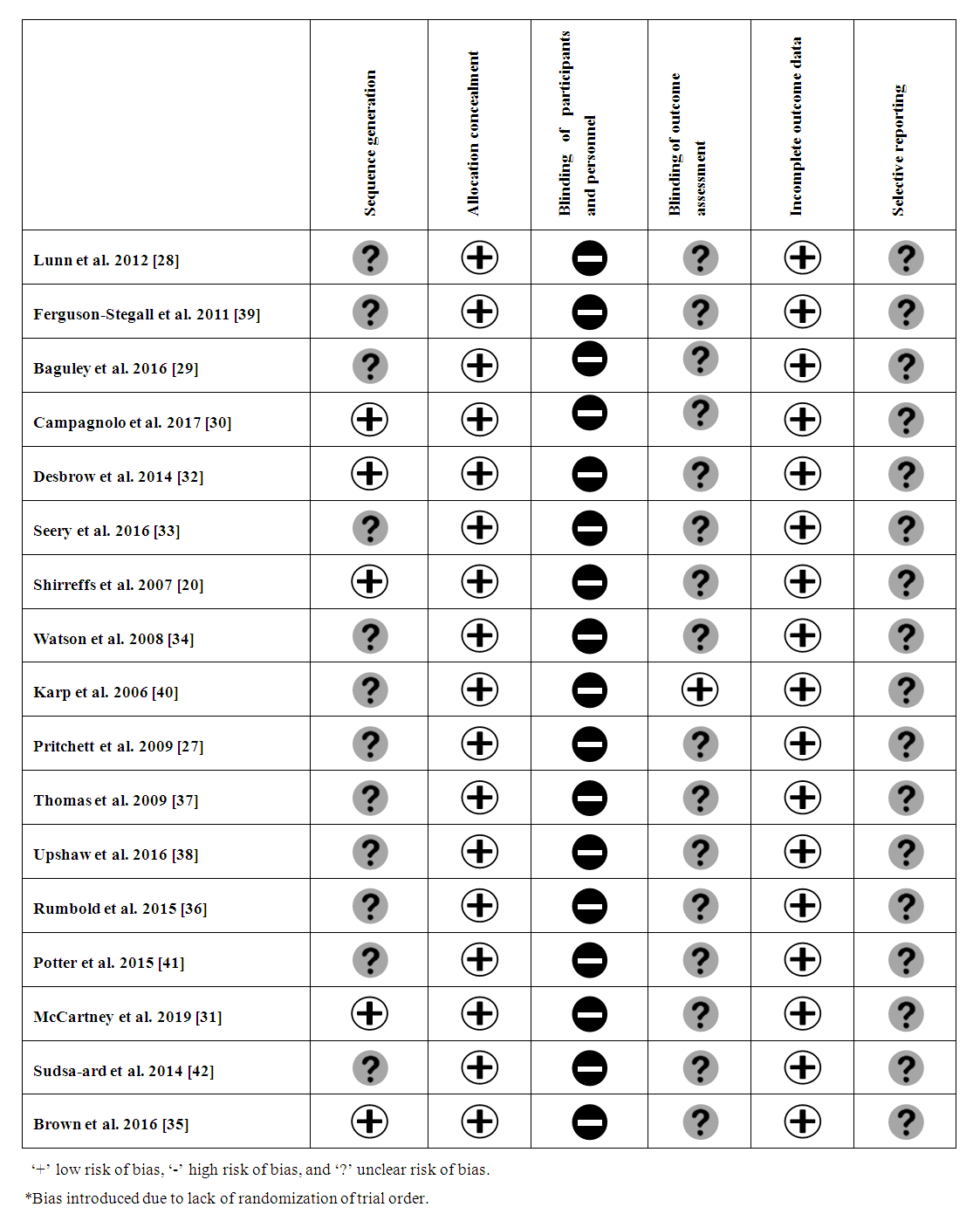
- Many studies did not specify blinding or randomisation procedures, therefore risk of performance and detection bias with or without order effect respectively, has been judged as ‘unclear’ (Figure 2).
4. Discussion
- The aim of the current SLR was to systematically identify and synthesize research that investigated the effect of dairy milk in comparison to alternative post-exercise beverages on markers of ‘exercise recovery optimisation’, including muscle protein synthesis, muscle glycogen resynthesis, hydration status, immune competency, gastrointestinal status, and endurance performance. Results from the current SLR found that dairy milk enhanced muscle protein synthesis (muscle protein synthesis (i.e., mixed fractional synthetic rate) and elicited similar rates of muscle glycogen resynthesis compared to an isocaloric non-nitrogenous carbohydrate replacement beverage. Moreover, a dairy milk or dairy milk-based beverage resulted in similar restoration of NFB compared to a CEB and water, when consumed ad libitum, and a more positive NFB when consumed in equal volumes. Five studies observed enhanced endurance performance with a dairy milk beverage compared to an isocaloric beverage, while two studies found no differences. To date, no study has investigated dairy milk consumption after endurance exercise on markers of immune competency, or gastrointestinal status. Based on the current SLR findings and additional research investigating the effect of carbohydrate, protein, and water provisions in the time period after endurance exercise, recommended nutritional intake for ‘exercise recovery optimisation’ is depicted in Figure 3, which is in accordance with the nutritional composition of CM reported in the inclusion studies.
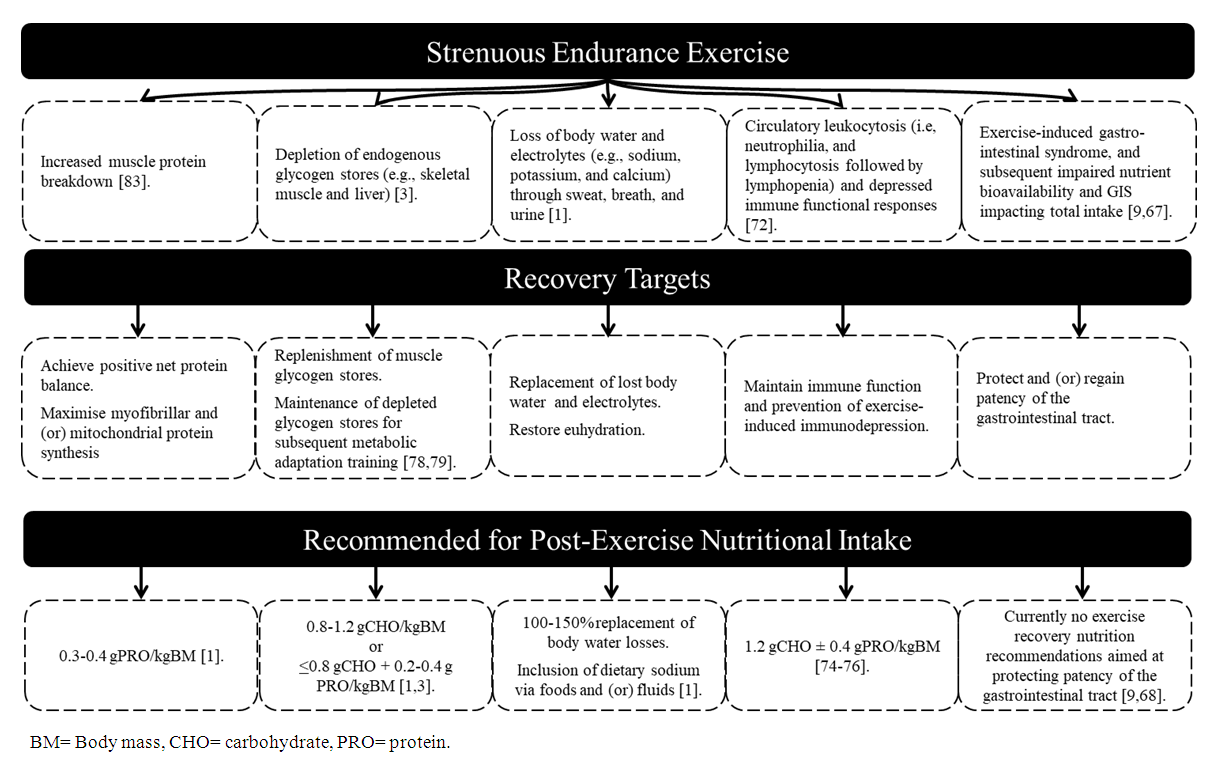 | Figure 3. Evidence based rational and justification for the nutritional composition aimed at ‘exercise recovery optimisation’ |
4.1. Muscle Protein Synthesis
- Skeletal muscle is a highly dynamic tissue and is the main site of training adaptations. Net protein is the difference between muscle protein synthesis and muscle protein breakdown. A positive net protein balance is required for muscle accretion and training adaptations, with both exercise stimulus and nutritional intake acting as a modulator of this balance [43-45]. The current SLR found that consuming dairy milk after endurance type exercise will enhance mixed muscle protein synthesis (i.e., myofibrillar and mitochondrial protein synthesis) compared to a non-nitrogenous carbohydrate beverage. These findings are not surprising given the role of dietary protein as a mechanistic stimulator and nitrogenous substrate for muscle protein synthesis [44,46,47]. The observed anabolic effect in the reviewed studies is likely due to the high essential amino acid (EAA) content of dairy milk, in particular leucine [48]. For example, increasing the concentration of plasma EAAs has been shown to stimulate muscle protein synthesis through the mTOR pathway, independent of exercise [49]. Both of the reviewed studies examined changes in the concentration of phosphorylated intramuscular signalling proteins along the mTOR signalling pathway, and observed increases in phosphorylation of translational signalling proteins after consumption of a dairy milk recovery beverage, including eIF4E-BP1, mTOR, and rp-S6 [28,39]. Such findings suggest that the protein quantity and quality of the dairy milk consumed after endurance exercise is a potent stimulator for muscle protein synthesis. While these findings provide evidence of an overall increase in muscle protein synthesis, the specific protein fractions (i.e., myofibrillar or mitochondrial proteins) in response to consumption of a dairy milk beverage after endurance exercise remains unclear. Adaptations to endurance exercise are characterised by an increase in the density of mitochondrial proteins, thereby increasing the oxidative capacity and metabolic fatigue resistance of the muscle. Endurance exercise has been shown to acutely stimulate mitochondrial protein synthesis to a greater degree than myofibrillar protein synthesis [50]. Results from this review suggest that the protein content of a dairy milk recovery beverage (0.2 g/kgBM/h) will stimulate overall muscle protein synthesis to a greater degree than a non-nitrogenous recovery beverage, however further research is required to elucidate the effect of a dairy milk beverage after endurance exercise on mitochondrial protein synthesis specifically.
4.2. Muscle Glycogen Resynthesis
- Replenishment of muscle glycogen stores has been established as a major determinant of endurance exercise performance during subsequent performance testing [51,52]. Therefore, failure to adequately replenish muscle glycogen stores is a major limiting factor for achieving optimal endurance performance, particularly when athletes must undertake multiple endurance training sessions or competitions with limited time for recovery [1]. It is recommended that high rates of carbohydrates (1.0 g/kgBM/h) are consumed during the acute recovery time period in order to maximise muscle glycogen replenishment [3]. Since the carbohydrate content of the dairy milk beverages provided in the reviewed studies did not meet these recommendations (0.6-0.8 g/kgBM/h), it appears that the protein content of the dairy milk beverages aided muscle glycogen resynthesis, as both studies observed comparable rates of resynthesis to isocaloric beverages with higher carbohydrate contents. Previous research, highlighted within several reviews, have established that when carbohydrate intake is less than 0.8 g/kgBM/h, provision of protein within the carbohydrate containing beverage, to compensate energy intake, will elicit similar rates of muscle glycogen resynthesis [53-55]. This is likely due to the heightened insulin response after consumption of combined carbohydrate and protein, which stimulates glucose uptake at the skeletal muscle plasma membrane (i.e., GLUT4 transporter) and glycogen synthase activity within the intracellular cytoplasm [56,57]. Therefore, results from this review suggest that coingestion of protein (e.g., 0.4 g/kgBM) with carbohydrate (e.g., ≥0.8 g/kgBM) provided by a dairy milk exercise recovery beverage will result in comparable rates of muscle glycogen resynthesis to an isocaloric CEB [57-59].
4.3. Hydration Status
- It appears that consumption of dairy milk or milk-based recovery beverage will result in comparable restoration of NFB to CEB and water when consumed ad libitum, and a more positive NFB when consumed in equal volumes. Previous research has demonstrated that fluid retention is enhanced with increasing sodium concentrations [60]. For trials in which hydration markers were measured after providing isovolumetric doses of each recovery beverage, the sodium content ranged from 18-59 mmol/L and 12-23 mmol/L for the dairy milk beverages and CEB, respectively. It is likely that the higher sodium and (or) the sucrose derived from added sugar in CM and MBSB (values not specified), may have contributed towards the superior restoration of fluid balance observed in the dairy milk trials [32,61].Additionally, the higher energy density of milk or milk-based beverages is likely to delay gastric emptying and intestinal transit, and consequently slow the delivery of water into circulation [62]. A slower rate of circulatory water delivery avoids acute and transient episodes of hypervolaemia, and subsequently attenuates the sudden drop in plasma osmolality, which leads to increased circulatory water clearance by the kidney through enhanced urine production and output. Three inclusion studies observed such responses, whereby POsmol was significantly lower during the water and (or) CEB trials than the milk trials, within 1 h of rehydration [31,33,34]. Additionally, increased urine output 1-2 h after rehydration was observed during the water and CEB trials, compared with dairy milk beverage trials [33,34]. Evidently, body water-electrolyte balance following strenuous exercise and fluid consumption behaviours are complex and dynamic in nature, and cannot be accurately measured with a single method. It has been suggested that ‘gold-standard’ for assessing hydration status at discrete time points in close temporal proximity under controlled laboratory conditions is a combination of TBW and POsmol [63]. Use of additional methods including ∆ PV, ∆ BM, urine measures of hydration, and thirst perception may help to provide a more comprehensive evaluation of body water dynamics during the acute recovery period [63,64]. Results from this current SLR suggest that provision of an isovolumetric dose of a dairy milk beverage after strenuous exercise will result in a more positive NFB, and a higher POsmol when compared to water and (or) a CEB. However, the effect of beverage differences on TBW was not investigated. Further research investigating the effects of a exercise recovery beverages on hydration status should utilise multiple methods including POsmol and TBW, at a minimum. Considering the technical and cost constrains of using an isotope tracer (e.g., deuterium oxide) with mass spectrometry techniques to determine TBW, the application of multi-frequency bioelectrical impedance analysis for TBW provides a practical alternative, bearing in mind the limitations of using such a technique [65,66]. However, it is imperative that such devices are validated against an isotope tracer technique, prior to application, thus ensuring accuracy and meaningful outcome interpretations.
4.4. Immune Function
- A single bout of prolonged and intense exercise may result in an acute immune disturbance, characterised by acute circulatory leukocytosis (e.g., neutrophilia and lymphocytosis followed by lymphopenia), reduced immune cell function (e.g., reduced bacterially stimulated neutrophil degranulation in-vitro, reduced mitogen or antigen-induced lymphocyte proliferation in-vitro, and reduced antigen-induced delayed hypersensitivity challenge in-vivo), perturbed oral-respiratory mucosal immunity, increased inflammatory cytokine responses [8,72-76]. While the clinical implications of such a response are inconclusive, it has been theorised that perturbed immune responses following a bout/s of endurance exercise, or during periods of intensified training, may decrease an athlete's resistance to the presentation of opportunistic invasion by pathogenic microorganisms, subsequently increasing risk for illness and (or) infection [72]. Therefore, targeted nutrition strategies to optimise recovery of immune function after and (or) between bouts of strenuous endurance exercise would appear to be a logical and favourable intervention. At present, there are no nutritional guidelines or recommendations that specifically focus on feeding post-exercise in order to support immune function. However, the consumption of an exercise recovery supplementation beverage containing 1.2 g/kg of carbohydrate with or without 0.4 g/kg of protein immediately following 2 h running at 75% V̇O2max has been shown to support post-exercise immune functional responses, as evidenced by a more favourable bacterially-stimulated neutrophil degranulation response and salivary antimicrobial status, compared with either a water control or delayed feeding (i.e., 1 h post-exercise) [74-76]. Considering dairy milk provides similar total carbohydrate and protein nutritional profiles per respective portion, it is plausible that dairy milk consumption immediately after strenuous endurance exercise may result in favourable immune outcomes, however this warrants substantiation [77].
4.5. Gastrointestinal Status and Intake Tolerance
- The term ‘exercise-induced gastrointestinal syndrome’ (EIGS) has recently been used to describe the perturbations to gastrointestinal epithelial integrity, gastrointestinal functional responses, local epithelial and systemic immune interactions that have been reported to present both clinical and performance implications [9,10,67,68]. The primary causal mechanisms of EIGS include altered circulatory- and neuroendocrine-gastrointestinal physiological processes in response to exertional stress, which results in secondary outcomes. These secondary outcomes may include, but not limited to: 1) decreased gastrointestinal motility, digestion, and absorption; 2) increased intestinal epithelial cell injury, increased intestinal epithelial tight-junction injury and (or) dysregulation, and subsequent hyperpermeability; and 3) increased local and systemic inflammatory responses. These outcomes have associations with extrinsic (e.g., exercise intensity, duration, modality, and heat exposure) and intrinsic (e.g., feeding tolerance, predisposition to gastrointestinal disease/disorder, and the gastrointestinal microbiota) exacerbation factors, and clearly linked to GIS that appears to be specific to the primary causal factors and secondary outcomes [9,68]. Such exercise-associated perturbations may have implications for post-exercise nutrition availability and feeding behaviours. Indeed, a growing number of recent studies have provided evidence of impaired absorption of carbohydrate and protein following exertional stress (i.e., both endurance and resistance exercise), likely due to exercise-associated enterocyte injury and (or) sympathetic drive suppressing submucosa plexus and brush border nutrient absorption influencing activities [8,10,11,69,70].Carbohydrate malabsorption of a pre-exercise carbohydrate-rich (120 g) meal has been observed in response to 2 h running at 70% V̇O2max in temperate ambient conditions with fluid restriction to induced 3.1% BM loss dehydration (breath H2 peak: 12 ppm) and with euhydration (breath H2 peak: 6 ppm) [8]. Meanwhile, 68% of an endurance athlete participant cohort demonstrated carbohydrate malabsorption of clinical significance (≥10 ppm breath H2) in response to 90 g/h consumption of a 2:1 glucose-fructose 10% wv during 2 h running at 60% V̇O2max, followed by a 1 h distance test, in temperate ambient conditions. These outcomes are not surprising considering reduced active and passive carbohydrate absorption has been reported in response to running at 70% V̇O2max compared to rest [69]. Moreover, a reduction in post-exercise protein absorption, as measured by in-vivo combination of 20 g L-[1-13C] phenylalanine labelled protein ingestion with continuous intravenous L-[ring-2H5] phenylalanine infusion, was observed after a single bout of resistance-type exercise [70].To date, no study has investigated the effect of exercise-associated gastrointestinal injury and functional impairment on dairy milk nutrient bioavailability in the post-exercise recovery period. However, given our current understanding of EIGS, it is possible that ingestion of dairy milk after strenuous endurance exercise may result in malabsorption of both carbohydrate and protein [71]. This may have implications for the recovery of perturbed physiological systems, from a nutrient bioavailability perspective; and may also induce GIS, further burdening feeding behaviours and nutrient provisions. For example, due to the relatively high energy density and lactose (i.e., a high fermentable carbohydrate) content of dairy milk, there is anecdotal belief that athletes may experience some abdominal discomfort. However, despite some studies reporting higher levels of bloating and (or) fullness with dairy milk consumption in the recovery period, this review did not find any consistent evidence of significant differences in abdominal discomfort when compared to alternative beverages.Finally, there is some evidence to suggest that consumption of dairy milk as a recovery beverage will reduce energy intake of a subsequent meal. It is, however, unclear if overall energy intake (i.e., total energy intake from the beverage and the meal) is offset. Further research in the area is required, as a reduction in overall energy intake following consumption of a dairy milk exercise recovery beverage might be a useful strategy for athletes who are aiming to create an energy deficit. Conversely, this strategy may be harmful to athletes struggling to achieve adequate energy intake.
4.6. Endurance Exercise Performance
- Four studies included in this review demonstrated that consumption of dairy milk as a recovery beverage resulted in an improvement in a subsequent protocol of exercise capacity or performance when compared to non-caloric alternatives [38,39,41,42]. This is not surprising given the importance of carbohydrate availability for prolonged endurance exercise performance or intermittent high-intensity exercise [1-3,6]. However, five studies also observed such improvement when dairy milk was compared to energy-matched alternatives [28,37,39,40,42]. The carbohydrate content of the dairy milk beverages in these trials were less than or equal to that of the comparator beverages. It can be theorised that the improved endurance exercise performance observed in these trials is attributed to the potential of dairy milk to stimulate MPS and aid rehydration, in addition to enhancing glycogen replenishment. Thus, dairy milk may provide a thorough nutritional base for ‘exercise recovery optimisation’, compared to the overall recovery outcomes when only one nutrient and (or) one recovery variable is targeted (e.g., carbohydrate or protein or water provisions on muscle glycogen resynthesis vs. muscle protein synthesis vs. rehydration vs. gastrointestinal and immune status).A final consideration around recovery and its link to longer term performance benefits involves the emerging evidence that withholding carbohydrate after a high quality training session might prolong the duration of the post-exercise period of increased transcription of adaptive proteins associated with endogenous fat energy substrate oxidation [78]. Undertaking endurance exercise with low endogenous glycogen stores appears to enhance activation of muscle intracellular signalling proteins (e.g., p38-MAPK, AMPK, and PGC-1α) that modulate the expression of enzymes and other proteins that effect mitochondrial biogenesis, fuel utilisation (e.g., increased fat oxidation at the respective exercise workload), and (or) aerobic economy and efficiency [78,79]. In addition, since the restoration of glycogen is inversely correlated with the increase in cellular signaling, the deliberate avoidance of carbohydrate in the acute recovery period may extend the period of enhanced adaptation [1,79]. It is, however, important to consider the frequency and placement of these dietary strategies. For example, low carbohydrate in the recovery period is not ideal when high intensity training sessions (e.g., HITT) or competition are consecutively planned, but may be more suited to promote adaptation to a high quality session when the subsequent workout involves moderate intensity exercise. As such, alteration of dairy milk to manipulate the carbohydrate content (e.g., addition of carbohydrate when the promotion of glycogen synthesis is desired, and membrane permeability manufacturer processing techniques inducing alteration in dairy milk lactose content for ‘recover low’ sessions) may add to the versatility of dairy milk as a recovery beverage.
4.7. Research Limitations, Future Implications, and Translational Application
- The authors acknowledge certain limitations to the translational applicability of the presented SLR findings. The potential language bias introduced through limiting the search strategy to English language papers only is acknowledged. Most studies included in the summary required participants to perform the exercise protocol in the fasted state. This is not representative of common training and competition practices, as athletes will often consume a meal or snack in the hours preceding exercise. Additionally, the majority of studies were conducted amongst male athletes. Given the well-established differences in body composition, metabolism, and physiological responses (i.e., hydration, gastrointestinal, and immune status in response to exertional stress) due to biological sex [10,66,80-82], these results might not reflect ideal recovery nutrition for female athletes. The intervention beverages have been categorised based on the general type of beverage (i.e., chocolate milk, milk based sports beverage, etc), and therefore have not considered the specific nutritional profiles of each beverage. Finally, due to the heterogeneity of the exercise protocols, nutritional composition of beverages and reported outcomes, a meta-analysis of the reviewed studies was not possible.Overall, the major limitation of all included studies, from the perspective of translational applicability, was the lack of universal or unifying markers of recovery with a transfer to a performance outcome. All studies focused on one or two key recovery components, with or without a subsequent endurance exercise capacity or performance test. Such experimental procedures are imperative for targeted assessment of a singular recovery cluster (i.e., markers for muscle protein synthesis, muscle glycogen resynthesis, or rehydration) with identification of outcomes and mechanisms of action advancing scientific knowledge. Nevertheless, a stronger investigation of the transfer to performance benefits is warranted to avoid misguided, misinterpreted, and (or) inappropriate applications of the research findings.Future applied research in relation to post-exercise nutrition should employ a comprehensive assessment of recovery markers (Figure 3), ensuring experimental designs are able to capture accurate interactions between the nutritional intervention/s and ‘exercise recovery optimisation’ outcomes. In particular, given the failure of previous research to take into account the magnitude of intestinal injury and impairment of nutrient transport associated with sympathetic drive early in the post-exercise period [8,71], it is likely that the potential for recovery may have been underestimated. Conversely, if the magnitude of EIGS is attenuated through pre- and (or) during-exercise nutritional or physiological manipulative strategies, then it is plausible that the nutrient bioavailability of the exercise recovery nutritional strategy will be enhanced [9,10,68,83,84].
5. Conclusions
- The nutritional properties of dairy milk beverages, particularly those with higher carbohydrate and sodium contents, closely align with the current nutrition guidelines for recovery from endurance exercise. Although several quality issues were identified across the included library, the current SLR provides evidence to suggest that dairy milk may provide equivalent or superior recovery of muscle protein synthesis, glycogen replenishment, and rehydration when compared to carbohydrate and carbohydrate-electrolyte alternatives, which appear to have performance benefits (e.g., dairy milk > non-caloric and caloric comparators). However, to date, there has been no research investigating the effects of dairy milk on immune and gastrointestinal status following strenuous endurance exercise.
ACKNOWLEDGEMENTS
- All authors contributed to the SLR process, preparation and review of the manuscript. IR, RC, JP and LB designed the search strategy. IR, VC and RC were responsible for screening, study selection and data extraction. IR and SG conducted risk of bias assessments.
DISCLOSURE
- The current SLR was supported by Lion Dairy & Drink Australia Pty Ltd. The funder had no involvement in the development of the search strategy, data extraction, synthesis or interpretation of results. No restrictions were placed on the reporting of SLR findings.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML