-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Sports Science
p-ISSN: 2169-8759 e-ISSN: 2169-8791
2019; 9(1): 17-23
doi:10.5923/j.sports.20190901.03

Malaria and Anemia Cause Impairments in Athletic Performance of Young Female Athletes Even after 1,5 Year of Clinical Healing
Ramón Nunez Cárdenas1, Ivete de Aquino Freire2, Yesica Nunez Pumariega3, Vernon Furtado da Silva4, Catalina Dominga Pumariega Torres5, João Rafael Valentim-Silva6, Edson dos Santos Farias7, Daniel Oliveira de Souza7, Mauro Tada7, Rodrigo Guerino Stabeli8
1Professor at Physical Education Department and Coordinator of the Center for Research in Sport of The Federal University of Rondônia (UNIR), Rondônia, Brazil
2Retired Professor from The Physical Education Department and Head of Research Group on Body Culture of The Federal University of Rondônia (UNIR), Rondônia, Brazil
3Master’s Degree Student in Psychology at The Federal University of Rondônia (UNIR), Rondônia, Brazil
4Visiting Professor at Physical Education Department of Federal University of Rondônia, Rondônia, Brazil (UNIR)
5Pedagogy Student at The Federal University of Rondônia (UNIR, Rondônia, Brazil
6Professor at Physical Education Department of The Federal University of Rondônia and the North Educational Union (UNINORTE), Acre, Brazil
7Professor at Physical Education Department of The Federal University of Rondônia (UNIR)
8Professor at the Faculty of Medicine of the Federal University of São Carlos (UFSCAR) and the Bi-institutional Institution for Medical Research at Fiocruz-USP, Campus of Ribeirão Preto – SP, Brazil
Correspondence to: João Rafael Valentim-Silva, Professor at Physical Education Department of The Federal University of Rondônia and the North Educational Union (UNINORTE), Acre, Brazil.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Malaria is a vector-borne parasitic tropical disease found in 91 countries and produce high levels of blood-stage parasites, severe infarcts in critical organs, and anemia. This condition may cause impairments in physical performance and in the athlete health, moreover, data about the impact of the malaria and anemia in performance is sparse. So, the purpose here was evaluate the physical performance of female athletes with a history of anemia and malaria infection even after one and half year of clinical healing. Ten female athletes with malaria and anemia history (GAM) and ten without this history (GNM), had haematological, biochemical, and performance parameters assessed and statistically compared. To the endurance the GNM showed high VO2Máx 23.56+1.55 mL/Kg.min than GAM 19.19+2.64 (p= 0.0016). To the abdominal strength the GNM showed more strength 56.8+8.35 than GAM 39.1+6.93 (p=0.0007), regarding the score of the arm strength test it became evident that the GNM also showed more strength 24.4+5.91 than the GAM 17.9+4.1 (p=0.0003). Still in relation to the stretching test the GNM had best performance 40.9+4.04 as compared to the GAM 29.5+3.68 (p=0.003), and to the speed variable the GNM with 9.02+0.45 seconds also evidenced a better performance than the GAM with 9.45+0.53 seconds (p= 0.0002). In conclusion, the malaria and/or anemia history of the athletes investigated here proved to cause impairment in their physical performance even considering the fact the disease was acquired by them one and half year before they were admitted to the research.
Keywords: Sports Medicine, Athletic Performance, Exercise Biochemistry, Chronic Disease, Physical Training, Parasitic Disease
Cite this paper: Ramón Nunez Cárdenas, Ivete de Aquino Freire, Yesica Nunez Pumariega, Vernon Furtado da Silva, Catalina Dominga Pumariega Torres, João Rafael Valentim-Silva, Edson dos Santos Farias, Daniel Oliveira de Souza, Mauro Tada, Rodrigo Guerino Stabeli, Malaria and Anemia Cause Impairments in Athletic Performance of Young Female Athletes Even after 1,5 Year of Clinical Healing, International Journal of Sports Science, Vol. 9 No. 1, 2019, pp. 17-23. doi: 10.5923/j.sports.20190901.03.
Article Outline
1. Introduction
- Malaria is a vector-borne parasitic tropical disease found in 91 countries worldwide. Plasmodium falciparum produces high levels of blood-stage parasites that promotes severe infarcts in critical organs in all age groups of those specimens causing severe anaemia to them. This disease is one of the major global diseases, being almost half of the world’s population at risk of contracting it. There is a close estimate that only in the 2016 year, close to 216 million of people would have contracted the parasite, being that 445.00 of the cases were expected to result in death [1].Historically, the city of Porto Velho is considered as being located at a high risks area for malaria. Although in the last couple years a reduction of the incidence of this disease has been observed, the incidence of malaria in that region still remains at an elevated risk of occurrence [1-3].Plasmodium sporozoites, when injected into the human dermis by a female anopheline mosquito, its use a molecular mechanism capable to rapidly enter the person circulatory system and translocate easily across the liver [4]. After invasion of hepatocytes, liver-resident parasites undergo asexual schizogony to form tens of thousands of merozoites. Following merozoites emerging from the infected liver cell, the parasites come to infect erythrocytes where they asexually replicate in circulation, and then leading to geometric population expansion and the consequent clinical symptoms of malaria. [5, 6].Malaria do not lead to severe acute complications in previously healthy individuals, but chronic infection has been described as an important cause of glomerular damage by deposition of immunocomplexes, producing a nephrotic syndrome [4], and the presence of anemia is commonly produced by intravascular hemolysis. Due to this, the immune fixation triggers a series of cascade reactions within the hemolysis of these cells [5], a condition that may impair a person physical performance in all the aspects of his/her commom life. In spite of this well recognized fact, the literature focusing on sport performance have not investigated it as related to athlete who contract and/or contracted this type of disease. For this study, the anemia variable is important and its relationship is explained by the rupture of the red blood cells as well as the difficulty to generate this kind of organic supplement. In Brazil this type of anemia is very common and its total incidence only is supplanted when compared to anemia caused by iron deficiency [2, 5, 6].In consonance to maintaining good health, success in sport is determined by the athlete's ability to maximize her/his genetic potential with an appropriate physical and mental training whose purpose is to prepare to competition [7, 8]. Athletic physical performance develops within a relative continuity in which the requirements are necessarily adequate to the organic capacity of the athlete, justifying a possibility that his/her performance may be chronically impaired at organic presence of malaria and/or anemia.In this concern, it should be clarified that compared to the general population, athletes, tend to have slightly lower hemoglobin concentrations a condition commonly known as "false anemia" [9]. This phenomenon occurs in function that in most programs of exercises considerable part of the training is practiced within aerobic characteristics, a factor of increase in blood plasma volume whose consequence is the dilution of the red blood cells and lowering in haemoglobin concentration. However, for individuals who suffered from malaria we may expect this pathologic condition could have different impact in their athletic performance, even considering that some studies indicate that a lower concentration of hemoglobin per unit of blood does not impair maximal physical performance [9]. It because in case of training for high performance the potential disadvantage of hemogoblin lowering can be offset by an increase in cardiac systolic volume caused by increased blood volume [10]. This discussion is very important to be considered by professionals engaged on research and exercise programs for sport performance, especially in the amazon region, were the prevalence of malaria is significantly high. As it was above presumed, the fact that malaria disease may affect the liver and red blood cells of an infected person and in being related with any type of exercise methabolism we may conceive that if conceived by an athlete its chronical effect may influence his/her perfromancve even many months after the clinical cure. This knowledge in countries were this desease is very prevalent like Africa, Brazil and more than 100 countries, can have crucial role in thletic training planning and athlete health manegement. Due this, the propose was evaluate the physical performance of female athletes with a history of anemia and malaria infection even before one year of clinical cure and to compare their performance with athletes without malaria and anemia historic.
2. Methods
2.1. Design and Participants
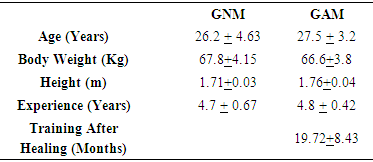
- The research is a descriptive-comparative study were twenty female athletes linked at two sports clubs in the city of Porto Velho were investigated. Within this group, 10 athletes with history of anemia and malaria and was called the GAM and 10 had no history of malaria and thus being called GNM. It must be emphasized that now of the study both groups were health. It because the GAM was treated and not had new incidence of malaria and anemia in the last 19 months after the begin of the experiments.The present study was approved by the Research Ethics Committee of the Health Center of the Federal University of Rondônia / CEP / NUSAU by registration number 002/2011/CEP of the Federal University of Rondônia. All participants in the study signed the informed consent form. The inclusion criteria were: (i) be federated athletes in the volleyball federation of the state of Rondônia, (ii) adults of the female gender, (iii) aged up to 18 years, (iv) residents in the municipality of Porto Velho and, (v) to agree to participate in the study by signing the Free and Informed Consent Form. In another side, about the exclusion criteria (i) did not agree to participate in the research, (ii) non-federated athletes in the volleyball federation of Rondônia, (iii) do not reside in the Municipality of Porto Velho and, (iv) athletes who receive contraindication of the Physician.Table 1 presents the characteristics of age group, gender, time of practice in the sport and weekly frequency of training of the athletes who pointed to anemia and malaria infection history. Table 1 presents the characteristics of age, gender, time of practice in sports and weekly training frequency of athletes who did not report a history of anemia and malaria infection.The table 1 show the characteristic of the sample of the group of athletes that pointed out anemia and malaria infection history.
|
2.2. Instruments and Proceedings
- For the process of data collection, a multidisciplinary team was formed, including professionals such as: physicians, physical educators, pedagogue, psychologist and nutritionist. A questionnaire was also used as a data collection instrument, aiming to retrieve a set of data and information related to the characterization of the investigated group. The questionnaire was tested in a small group to verify the language difficulties, understanding of the questions, time of application, receptivity of the athletes, time needed for training.
2.3. Physical Evaluation
- A 10-minute dynamic warm-up composed by jogging, dynamic upper and lower limbs movement drills including marching, marching toe touches, and hurdle step overs was performed by subjects at the beginning of each session. To evaluate the max aerobic power acquisition was applied the 12 min test of Cooper. The obtained max distance in twelve minutes are inserted in an equation in order the calculi the aerobic power.For the evaluation of the concentric isotonic strength of sports subjects was used the Trunk flexion test as previously described [11]. For all trunk flexion testing, participants were positioned supine, with both hips and knees flexed to 90 degrees, trunk inclined at 60 degrees resting on a prefabricated wedge. Stabilization was achieved with a belt around the table and over the dorsum of the feet (with shoes on) for the standard method. Participants crossed their arms across the chest, placing their hands on opposite shoulders, in a manner comfortable to them. Participants maintained their body position for as long as possible after the wedge was moved back 10 cm. Time was measured from the instant the prefabricated wedge was moved back until the participant visually re-established contact with the wedge. This was the same for all methods.Determination of the maximal strenght was assessed by using a maximum repetition (IRM) protocol done on leg and arm extension machines. A standard 1RM testing protocol was used for the assessment of the 1RM as outlined by the National Strength and Conditioning Association [12]. For the tasks, the subjects warmed-up with a light resistance that easily allowed 5 to 10 repetitions. Following a 1-minute rest, 4 to 9 kg was added, and the subject attempted to complete 3 to 5 repetitions. After a 2-minute rest, another 4 to 9 kg was added, and the subject attempted to complete 2 to 3 repetitions. Following a 4-minute rest, another 4 to 9 kg was added, and the subject attempted 1 repetition. If the attempt was successful, a 2 to 4-minute rest was given, and more weight was added, based on the subject’s judgment, until the subject could not complete one repetition. To measure quadriceps strength, the subject sat on the Leg Extension machine with the knee flexed at a 90° angle (full extension = O), with the lever arm of the device resting on the tibia of the lifting leg just proximal to the malleoli. To measure hamstring strength, the subject lay prone on the Leg Curl machine, with the lever arm across the Achilles tendon of the lifting leg. The same proceedings were used to evaluate the arm isotonic force, but, the ankle was measured in the elbow joint between the arm and the forearm. This proceeding was previously described [13].To evaluate flexibility the Trunk flexion test (Barrow and McGeeb, 2003) was used as previously described [14]. Finally, to evaluate velocity, the 50 m velocity test. The test involves running a single maximum sprint over 50 meters, with the time recorded. A thorough warm up should be given, including some practice starts and accelerations. Start from a stationary standing position (hands cannot touch the ground), with one foot in front of the other. The front foot must be behind the starting line. Once the subject is ready and motionless, the starter gives the instructions "set" then "go.” The tester should provide hints for maximizing speed (such as keeping low, driving hard with the arms and legs) and the participant should be encouraged to not slow down before crossing the finish line [15].
2.4. Blood Sample
- Two blood samples were collected at rest. Blood samples were collected in K3 EDTA tubes (3 mL vacutainer tube; BD Vacutainer, Franklin Lakes, NJ, USA) and stored at room temperature until measurement, which was performed in all cases within 1 h after venepuncture. Hematological parameters were analysed using the hem autoanalyzer (Siemens, A.G.; Erlangen, Germany) according to the manufacturer’s instructions. Haematocrit (%), hemoglobin (g dL-1), red cell count (1012 L-1), mean cell hemoglobin (pg), and Glucoses.Was done the quantitation of Alanine Amino Transferase (ALT), Aspartate Amino Transferase (AST), Urea, Creatinine, Bilirubin, was performed using a Kone lab 60i model automation and Wiener Lab kits following the protocol of the apparatus. Said automator was calibrated prior to measurements according to the manufacturer's manual. All experiments were performed in duplicate.
2.5. Statistical Analysis
- For the statistical analysis the data were described by means, standard deviation, the % was calculated. To evaluate the normality, the Kolmogorov-Smirnov test was used and, the data were considerated normal. The difference between the groups, the paired t student test was used with significance set at 5% (p <0.05) and the confidence interval was exibet. All procedures were performed in PrismStat 5.0.
3. Results
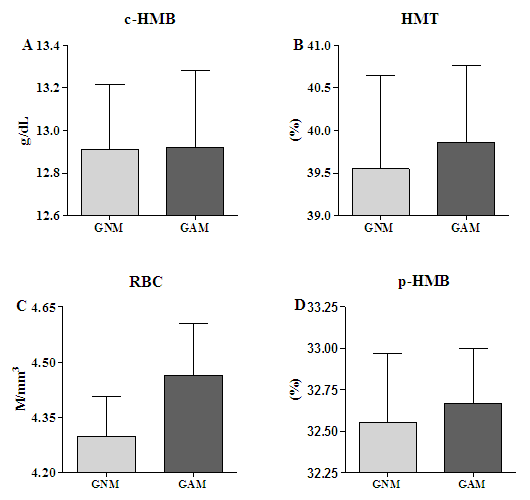
3.1. The Hematological Profile Showed Equal Red Blood Cell Profile among GNM and GAM
- The hematological parameters showed within the physiological variation and, to all comparison no difference (p>0.05) was observed (Fig. 1). To the absolute hemoglobin count the GNM showed 12.91±0.95g/dl and GAM 12.92±1.15 g/dl (Fig. 1A), to hematrocrit the GNM showed 39.55±3.47% and the GAM 39.86±2.84% (Fig. 1B). In relationship to the red blood cells count the GNM showed 4.29±0.34 million/mm3 and GAM 4.46±0.44milion/mm3 (Fig. 1C), and finally to hemoglobin concentration the GNM showed 32.55±1.32% and GAM 32.66±1.03% (Fig. 1D).
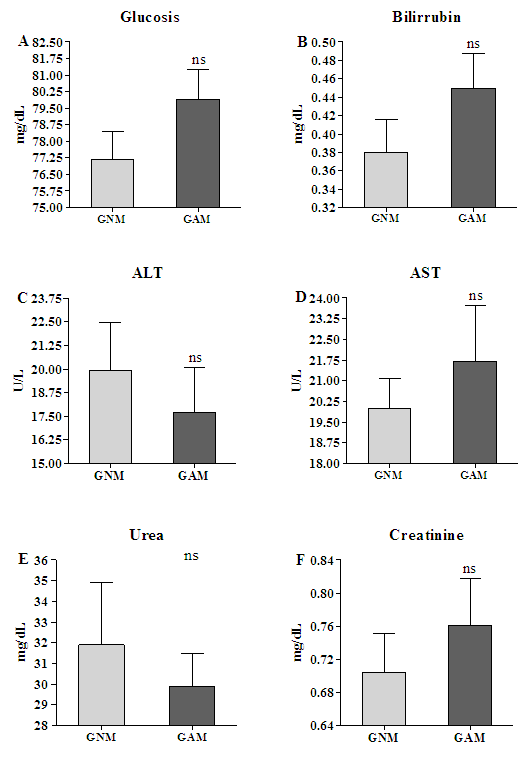
3.2. The Biochemical Assays Showed Equal Glucose Level, Hepatic, and Nephric Enzyme Profile
- The biochemistry showed that, to the parameters here assessed, both groups are in the normal physiological variability. The glucoses level was equal between GNM 77.2±3.9mg/dL and GAM 79.9±4.2mg/dL (Fig. 2A) (p=0.13). About the hepatic enzymatic profile the Bilirubin of the GNM 0.38±0.11mg/dL was equal of the GAM 0.45±0.11mg/dL (Fig.2B) (p=0.06), the ALT of the GNM 19.92±7.9U/I was equal of the GAM 17.7±7.4U/I (Fig. 2C) (p=0.63), and the AST of the GNM 20±3.4U/I was equal of the GAM 21.7±6.4U/I (Fig. 2D) (p=0.45). Finally, to the renal enzyme profile the urea of the GNM was 31.9±9.5mg/dL, and of the GAM was 29.9±4.9mg/dL (Fig. 2E) (p=0.42), and, the creatinine of the GNM 0.7±0.1mg/dL, and of the GAM 0.76±0.17mg/dL (Fig. 2F) (p=0.49).
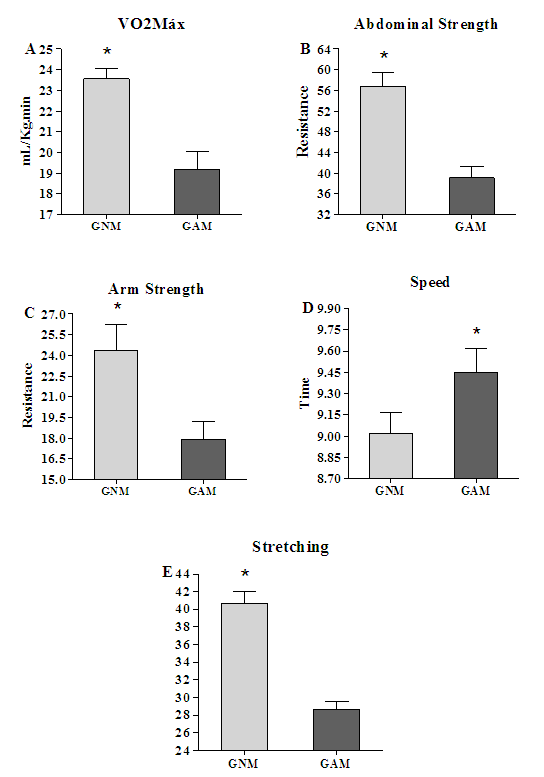
3.3. The Athletic Performance was Different between GNM and GAM
- To the endurance the GNM showed high VO2Máx 23.56±1.55 mL/Kg.min than GAM 19.19±2.64 with interval confidence (95%) from "3.216 to 5.530" (p= 0.0016) (Fig. 3A). To the abdominal strength the GNM showed more strength 56.8±8.35 than GAM 39.1±6.93 with interval confidence (95%) from "14.66 to 20.74" (p=0.0007) (Fig 3B). Looking at the score of the arm strength test we see that the GNM also showed more strength 24.4+5.91 than GAM 17.9±4.1 with interval confidence (95%) from "4.387 to 8.613" (p=0.0003) (Fig. 3C). In relation to the stretching test the GNM had best performance 40.9±4.04 than GAM 29.5+3.68 with interval confidence (95%) from "7.635 to 16.37" (p=0.003) (Fig. 3E). In a same way, for the speed variable the GNM had better performance than the GAM, being the score for the first, 9.02±0.45 and 9.45±0.53 with interval confidence (95%) from "-0.5919 to -0.2681" for the second (p= 0.0002) indicating the GAM as taking more time to cover a same distance as did the GNM (Fig. 3D).
4. Discussion
- The present study attempted to add some knowledge about the effect of the malaria and anemia upon the physical performance of athletes. To accomplish this objective, several experiments were carried out to analyse athletes who had and others who had not malaria. Our main data show that malaria and/or anemia may explain the decrease of physical performance of the volleyball athletes, here studied. It because athletes with a history of anemia and malaria demonstrated as having lower physical performance than the healthy ones even though the weekly training frequency manipulated, for both groups, was the same and parallelly their blood count had no considerable difference between them. Analysing individually the physical variables studied, we verified that both groups had low score in the physical resistance, a variable that might be considered basic for the performance of all types of sports. Female professional athletes of high performance level should resort distance of 3000 meters in 12 minutes and all athletes, of our two groups, did not reach that mark [16]. As it can be seen in Figure 3-A the group of athletes with non-malaria and/or anemia history (GNM) reached an average distance of only1,368 meters, performance which according to the literature in this area, equivale to 45.6% of the total distance to be covered by athletes at this level of training in this type of test. In a typical seemed difficulty, the GAM covered still less distance with only 1.550 meters, course equivalent to 51,5% of the suggested distance for high level athletes. It indicates that both groups had had enormous difficulty to develop velocity, a fact that may be related to a bad training program or organics deficiencies which should not be common for well-prepared athletes. Although the data here exposed can be seem as very normal in consideration to the two investigated groups, there was an improbable result which relates to the stretching test in which the GNM had a better result as compared to the GMA, since the expected result would be of an equal performance between them. It because there is no plausible evidence in the literature that could explain the reason why a disease such malaria would directly affect muscle stretching. The results obtained in "arm strength" in both groups are not favourable from the point of view of physical conditioning, although a better result is seen in the GNM. It because to be considered with excellent preparation in this physical quality, athletes aged 20-29 years old should reach more than 49 repetitions in flexion and extension of arm until freely interrupting the exercise. In the present case the GMN reached 18 repetitions, corresponding to 36.7% of recommended in the literature [14]. On the other hand, the healthy athletes performed 25 repetitions, which corresponds to 51% of the total, prescribed by the literature in this area [14]. A deficiency in performance of this physical quality may affect the efficiency of offensive technical fundamentals for success in the sport which they are practitioners. For abdominal strength the performance of the investigated groups was also seen as corresponding to a very low level. The optimal evaluation of female athletes in the variable abdominal strength is of 98-115 repetitions. Comparatively the group of athletes with a history of anemia and malaria had a mean of 40 repetitions, corresponding to 34.7% of recommended as ideal; healthy athletes presented 56 repetitions; equivalent to 48.6% of the total recommended in the test evaluation. Deficiency in the development of abdominal strength can affect body posture during the execution of the different technical and tactical foundations that are performed in this sport, causing premature fatigue, the risk of sports injuries, and low performance in several of its inherent fundamentals.To the stretching test, the female athletes granted an excellent evaluation, since to be in a top level in this physical quality an athlete should reach a joint amplitude of 25cm when at 18-25 years and 24cm at 26-35 years old. The performance of the investigated groups in this physical quality was 29cm to GNM and 39cm to GAM. As it has been shown in the related literature, this physical quality depends on the joint amplitude of the tested athlete [17-19]. As it was not a variable under investigation here, the cause of the good performance of the GNM and the GAM was not thought as being necessary for clarification.In the speed test the athletes in the GAM reached 9.45 seconds while the GNM obtained 9.0 seconds, evidencing a notable difference between both. The literature in this area makes clear that a good prepared athlete must have a time less than 5.8 seconds [20, 21], fact that indicate the two groups, the GAM and the GNM, as being in one scale of inadequate physical preparation. It also became evident the existence of difference of physical fitness between them, with a clear advantage to the GNM one. Perhaps a more plausible explanation to the low performance of the two group on this test would be linked to the training program they had had. The advantage of the GNM upon the GAM, may be speculated as an effect of malaria upon the health of the GAM, although the disease was treated for all athletes in this group and no evidence of the anemia was observed in the their hemograms. In another side, there is still some room to associate another hypothesis to explain the GNM speed advantage by linking the alterations that the hepatic phase of malaria may produce in glucose metabolism of a person affect by it (a non-investigated hypothesis) [22-24].In sum all the data show the athletes without malaria and anemia history as having better performance than the athletes with malaria and anemia history in all the applied tests. Besides it stood clear that considering the phase of physical preparation in which all of them were, the level of physical condition they demonstrated was below that expected for a team of volleyball players, even for the group that did not had malaria and anemia. However, the low performance level of the GMA, as compared to the GNM one, in all the tests, may serve as a reasonable indicative for admitting the hypothesis that a historic of malaria can be a minimizing factor for physical conditioning gains. These results must be understood as being of high relevance since the risk for acquiring malaria in the Porto Velho region remains high, including for the people who lives in the urban area. Science is an instrument for physical educators, coaches, and other professionals who must prepare athletes to maximum healthy physical conditions at all levels of a competition. Sportive practice is increasing in this north region of the country and the level of inter-sport competition is becoming higher at each day. Knowing about the limitations that certain types of disease and/or syndromes with major prevalence within a region may impact an athlete performance is an efficient instrument and challenge for all professionals engaged in sports at all levels, mainly those who works in regions infected by parasitic diseases.In perspective, it is suggested that to advance knowledge in the relationship between malaria and physical performance it will be necessary to add new insights into the knowledge of the mechanisms that interact with that disease and/or anemia. Although there may exist several metabolic interactions influencing physical conditioning and consequent athletic performance, seeing upon those linked to glucose metabolism, capacity of the haemoglobin saturation and oxygen transport may be a promising start.
ACKNOWLEDGEMENTS
- To the volunteers who graciously participation.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML