-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Sports Science
p-ISSN: 2169-8759 e-ISSN: 2169-8791
2018; 8(5): 145-151
doi:10.5923/j.sports.20180805.02

Effect of Short, Strenuous Exercise on Salivary IgA Levels in Obese Males
Elías Torres García1, Patricia Concepción García Suarez1, Iván Rentería1, Peter Walter Grandjean2, Alberto Jiménez-Maldonado1
1Facultad de Deportes, Universidad Autónoma de Baja California Campus Ensenada, Baja California, Mexico
2Department of Health, Human Performance and Recreation, Baylor University, Waco, Texas, U.S.A.
Correspondence to: Alberto Jiménez-Maldonado, Facultad de Deportes, Universidad Autónoma de Baja California Campus Ensenada, Baja California, Mexico.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

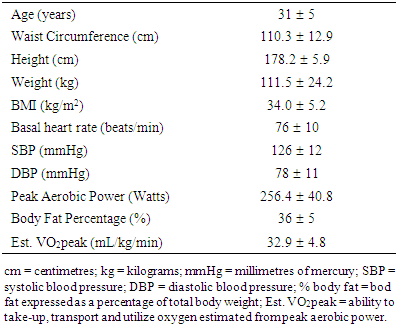
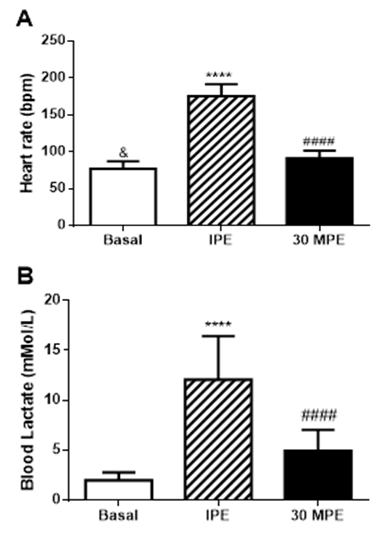
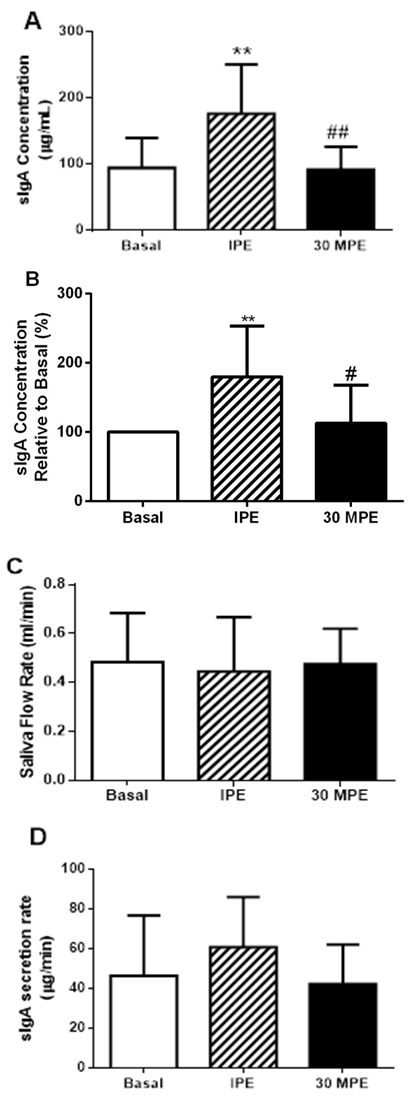
Obesity and strenuous exercise are independent stimuli with potential to modify immune function in opposition. Our purpose was to investigate transient salivary secretory immunoglobulin A concentration [sIgA] responses to short, strenuous exercise - a maximal graded exercise test (GXT) – in physically active, obese men. Eleven obese (35.5 ± 5.0 % body fat), healthy men performed a GXT on a mechanically-braked cycle ergometer. Unstimulated saliva was collected in basal state (Basal), immediately post-exercise (IPE), and 30 minutes post-exercise (30MPE). Heart rate and blood lactate concentrations were evaluated at the same time as saliva collection. Salivary [sIgA] was determined by ELISA and total protein concentration by Biuret assay. Saliva flow rate (SFR) was determined by dividing the total volume of saliva by the collection time in minutes. sIgA secretion rates were estimated using [sIgA] and SFR. During the GXT, heart rate increased from 77 ± 10 bpm at Basal measurement to 176 ± 16 bpm at IPE and remained elevated (92 ± 10 bpm) at 30 MPE (p < 0.0001). Blood lactate increased from 1.9 ± 0.7 to 12.1 ± 4.3 mMol/L at IPE and stayed elevated (4.9 ± 2.1 mMol/L) at 30MPE (p < 0.0001). Salivary [sIgA] increased by an average 81.7 μg/mL (~80%) from Basal (94.2 ± 45.0) at IPE and returned to basal levels (91.4 ± 34.5 μg/mL) at 30MPE (p= 0.003). Salivary sIgA secretion rate increased 73.5%; however, this response was not statistically significant (p = 0.11). Salivary protein and SFR remained unchanged after GXT. These findings provide evidence that short, strenuous exercise enhances, rather than suppresses, respiratory immune function in healthy obese males. SFR and sIgA secretion rates do not fully explain the higher post-exercise salivary sIgA observed in this study.
Keywords: Secretory immunoglobulin A, Obesity, Strenuous exercise, Blood lactate, Heart rate
Cite this paper: Elías Torres García, Patricia Concepción García Suarez, Iván Rentería, Peter Walter Grandjean, Alberto Jiménez-Maldonado, Effect of Short, Strenuous Exercise on Salivary IgA Levels in Obese Males, International Journal of Sports Science, Vol. 8 No. 5, 2018, pp. 145-151. doi: 10.5923/j.sports.20180805.02.
1. Introduction
- The prevalence of obesity has increased over the last century and particularly in recent decades [1, 2] and is recognized as a public health problem worldwide [1]. Obesity alters metabolic and endocrine factors [2] and increases the risk of acquiring diseases such as diabetes, hypertension, cardiometabolic diseases, and dyslipidemia [3]. Furthermore, obesity appears to induce changes in both innate and acquired immunity system [4]. When compared to their normal weight counterparts, obese individuals have higher total leucocytes counts, higher monocyte and granulocyte phagocytosis but lower T and B cell function [5]. Frasca and colleagues observed modifications in B cell phenotype in both, young and elderly obese [6]. Obesity appears to influence innate immunity by lowering natural killer sensitivity to its endogenous inhibitor such as cortisol in obese people compared with non-obese subjects [4]. Secretory immunoglobulin A [sIgA] is a predominant immunoglobulin in secretions of the mucosal immune system [7]. In saliva, sIgA is considered the first line of defense against harmful pathogens [8]. Namely, sIgA inhibits the attachment and replication of pathogenic microorganisms, preventing pathogens colonization on mucosal surface [9]. The sIgA’s role on the mucosal immune system is supported by the scientific evidences that showed a strong relationship between the lower salivary sIgA levels and the higher incidence of the upper respiratory tract infections (URTI) in athletes and non-athletes [10, 11]. Although a pathogen’s presence is the principal factor to induce changes in sIgA concentrations, other factors such as age [12, 13], caffeine ingestion [14] and physical exercise [15-17] also modify the sIgA levels in the saliva. With respect to physical exercise, studies performed in young adults show acute exercise increases sIgA [18, 19], reduces sIgA [7, 20] or does not change sIgA concentrations [21]. The controversies appear to stem from several factors such as fitness levels, exercise modes, intensities and fidelity of sample collection. Moreover, previous work to determine immune function responses after acute exercise were performed in normal weight individuals. Few studies have been designed to determine the influence of exercise on immune function in obese humans, and particularly, on sIgA levels. [22-24]. Obesity is a condition associated with alterations of nutritional, metabolic and endocrine factors which seem to be involved with an immunologic deficiency [2]. Given the powerful influence of excess body fat on several health parameters, it seems of vital importance to characterize acute effects of exercise on innate immune system responses in obese humans. The principal aim of the present study was to determine the effect of short, strenuous exercise on saliva sIgA kinetics in physically active obese men. It was hypothesized that short and strenuous exercise would decrease transiently salivary sIgA in obese men.
2. Methods
- Participants Eleven obese, apparently healthy men were recruited in the present study. All participants received general information about the aims of study, the experimental procedures, and gave their written consent to participate. This study was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the institutional Bioethics Committee of the Research Department of Universidad Autónoma de Baja California (IRB’s protocol 431/465/E). All participants in the present study were involved in regular, non-professional sports training programs such as football, and soccer. The subjects reported in the International Physical Activity Questionnaire (IPAQ) training three times a week at moderate to vigorous intensity for 60- 90 minutes. The average number of years they had been practicing the sport was 10. Experimental Procedure All participants attended the laboratory on two occasions between 8:00 and 11:00 AM. During the first occasion, the investigators explained in detail the methodology to perform the graded exercise test (GXT), and the procedures to collect the saliva samples. A physician performed a medical screening to exclude the possibility that participants were experiencing an acute respiratory tract infection at start the study. Participants were asked to refrain from strenuous exercise 24 hours prior to anthropometric measurements and undergoing the GXT. In addition, participants were asked to avoid alcohol, caffeine and acidic or high-sugar foods for 12 hours before the GXT. During the second occasion (24 hours after the medical screening), the participants arrived at the laboratory with a two-hour fast and underwent anthropometric measurements. Next, participants completed the GXT. Blood lactate concentration [La] and heart rate were determined in basal resting state (Basal), immediately post-exercise (IPE) and again at 30 minutes post-exercise (30MPE). Unstimulated saliva samples were collected at all three collection times (Supplementary Figure). Anthropometrics Measurements Height was evaluated with a digital stadiometer (BSM170, Biospace, Seoul, Korea). An electronic balance (Rice Lake, Floor Level) was used to determine body weight. Body mass index (BMI) was calculated by the formula: weight (kg)/height (m2). Waist circumference (WC) was measured at the level of the minimal waist, at the end of the normal expiration [25]. Bioelectrical Impedance Analysis, using the InBody770 (Cerritos, CA. USA), was employed to determine fat percentage of total body weight in accordance with the manufacturer’s instructions. Cardiovascular and Metabolic VariablesImmediately following anthropometric measurements and prior to the GXT, participants were seated in a comfortable position for 5 min. Heart rate was measured with a Polar FT1 heart rate monitor at rest and throughout the exercise and recovery periods. Additionally blood pressure was determined manually using stethoscope and sphygmomanometer and [La] was measured from finger capillary samples (Nova Biomedical lactate analyser) following the methodology reported by others [26, 27]. Saliva CollectionParticipants were instructed to rinse their mouth 3 times for 30 sec with distilled water and then were seated in comfortable position with their heads leaning forward and slightly lowered for 5 minutes. After 5 min of seated rest, unstimulated whole saliva was collected following the methodology previously described by Baralic et al. [7]. Participants expectorated three times during 2 minutes at 0, 60 and 120 sec into a sterile 15 mL Falcon tube. Participants performed the same procedure to collect saliva samples at IPE and 30 MPE; however, at IPE, there was not a resting period. Saliva flow rate (SFR) was determined by dividing the total volume of saliva (mL) by the collection time in minutes [18]. After the SFR was measured, samples were stored at -20°C for future total protein and sIgA analysis. Graded Exercise TestParticipants performed a GXT on an electro-mechanically braked bicycle ergometer (Lode Excalibur Sport 925900) following the methodology described by Storer et al. and Guiraud et al. with some modifications [28, 29]. Participants were instructed to stay seated on the cycle ergometer trough the test. The GXT started with a 4-min warm-up period at 20 W. At the fifth minute, the workload was set to 60 W. After this time, the workload increased by 20 W every minute until exhaustion or maximal effort. Subjects were verbally encouraged by the test administrators to provide a true maximal effort. Through the GXT, participants were required to maintain a pedal cadence between 70 and 80 rpm. The GXT was finished when participants could no longer maintain the pedal cadence of 70 rpm for 10 sec. The power of the last complete stage was considered as maximal aerobic power. The maximal heart rate was registered and maximal oxygen uptake was estimated indirectly with the methodology described by Storer et al. [29]. Exercise intensity at the end of the GXT was calculated using the formula (heart rate at the end GXT/estimated maximum heart rate) *100. Estimated maximum heart rate was calculated using the formula described by Tanaka (2001) (208- 0.7 X age), as this equation appears to be more accurate for overweight and obese people [30].Saliva Analysis Thawed salivary samples were measured for sIgA concentrations using an ELISA kit (Salimetrics, LLC, Carlsbad, CA) according to the manufacturer’s instructions. To avoid interassay variability, all the samples from each participant were assayed on the same microtiter plate. The limit of sensitivity for sIgA was 2.5 μg/mL. Color was detected 10 min after the stop solution was added and the absorbance was read at 490 nm with a microplate absorbance reader (iMark BIO-RAD, Hercules, CA, U.S.A.). The sIgA secretion rate (μg/min) was determined by multiplying the absolute sIgA concentration (μg/mL) with the SFR (mL/min) (Sari-Sarraf et al. 2007). Total salivary protein were determined by the Biuret protein assay (Spinreact, Girona, Spain). Bovine serum albumin was used as standard following the manufacturer’s instructions. In the Biuret assay, all the samples were analyzed at the same time.Statistical Analysis Dependent variables of interest were salivary [sIgA], total salivary protein, SFR, sIgA secretion rate, [La], and heart rate. Comparisons across time were made using multiple one-way analysis of variance (ANOVA) with repeated measures for sampling time (pre-exercise basal measurements = Basal; immediately post-exercise = IPE, and; 30 minutes post-exercise = 30MPE). Tukey’s post hoc test was employed to follow-up significant ANOVA results. Measurements at post-exercise sampling points were compared to Basal and were considered to be significant at p < 0.05. Additionally, to assess the magnitudes of the differences between Basal, IPE and 30 MPE the Cohen’s d was employed, confidence interval was set at 95%. Statistical analyses and graphs were made with GraphPad Prism 6.0 software [31].
3. Results
- Anthropometric and cardiovascular variables at baseline are displayed in the Table 1. BMI and waist circumference agree with anthropometrics references described by the National Institute of Health (NIH 1998) to classify our participants as obese.
|
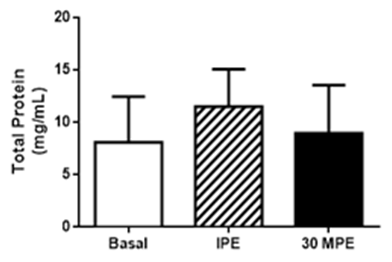 | Figure 3. Salivary total protein responses to GXT. Basal = pre-exercise; IPE = immediately post-exercise; 30MPE = 30 min post-exercise. All data is presented as Mean + Standard Deviation |
4. Discussion
- The present study assessed that a single episode of strenuous exercise, performed at maximal or near maximal effort, transiently increased salivary [sIgA] in obese men. These results does not meet with the initial hypothesis of the study. The exercise effect seems to be specific of sIgA, because the total protein concentration in saliva did not change with the GXT. Although sIgA secretion rate responses did not reach statistical significance, the effect was large (e.g., 73% increase and a Cohen’s d = 1.12), suggesting that sIgA secretion rate may play a role in the greater post-exercise [sIgA] observed in physically active, obese men. Graded exercise testing is typically performed in treadmill or cycle-ergometer to determine aerobic fitness [29]. Continuously-graded tests are designed to increase exercise intensity in a systematic fashion over 9 to 12 minutes until the individual is unable to maintain the work rate [32]. Participants in the current study appeared to achieve a maximal or near-maximal effort because they were unable to maintain exercise demands at the end of the test. Maximum heart rate represented > 90% of age-predicted heart rate max. Blood [La] also responded in a manner that would indicate strenuous physical effort (e.g., ≥10 mMol/L). These data are consistent that the subjects performed a maximal effort during the GXT [33]. Although, reference values for sIgA does not exist; the observed basal [sIgA] of 94.2 μg/mL are within ranges reported elsewhere for healthy males [19], [34-36]. Even though basal [sIgA] showed a wide variability, ranging from 20 μg/mL to 200 μg/mL [19, 34], we may postulate that the present cohort exhibited healthy mucosal immune function. Others have reported transient increases in [sIgA] following exercise [19]; however, we are one of the first, if not the first, to report a single episode of strenuous exercise of maximal effort transiently increases [sIgA] in obese men. The presented data contrast with others who report lower [sIgA] immediately after GXT in lean endurance athletes [35]. In addition to body composition, exercise training status and fitness levels, a difference between our current findings and those of Allgrove et al. [19] and Ihalainen et al. [35] was the mode of exercise – cycle ergometer vs. treadmill. At present, it is not readily apparent why engaging in weight-bearing, non-jarring exercise would produce different [sIgA] responses as compared to weight-bearing and jarring exercise. Fortunately, additional investigation as to the influence of training status, fitness levels, body composition, exercise mode and intensity on sIgA and respiratory immune function are fairly straight-forward and would help address differences between acute respiratory immune responses and more gradual adaptation. The molecular mechanism to explain higher [sIgA] has not been completely elucidated. One hypothesis is that lower [sIgA] after the strenuous exercise is a consequence of greater sympathetic activity, which may reduce saliva flow rate and negatively affects sIgA secretion and [sIgA] [9], [12]. Yet, SFR remained unaltered and it is not clear how much of an influence the sIgA secretion response played in increasing [sIgA] following the GXT in the current study. As such, these variables may not fully explain the higher [sIgA] in our study. Basic research performed in rodents, have demonstrated that the sympathetic activation increases [sIgA] concentration [37-39]. Some suggest that sympathetic activity increases the transcytosis of polymeric immunoglobulin receptor (pIgR) on the epithelial cells that finished in a higher salivary [sIgA] [38]. Others indicate that the sympathetic activity stimulates pIgR and secretory component levels in the salivary glands [37]. Recent work demonstrated that norepinephrine activates α and β receptors on the submandibular glands to increase the secretion of sIgA, although this effect is dependent of the pIgR expression and [sIgA] inside the cells [39]. Greater pIgR expression and [sIgA] were found during daylight time [39]. Resting heart rate is governed predominantly by vagal (parasympathetic) activity [40], as the exercise intensity increases, the sympathetic activity stimulates heart rate and is reflected in a higher heart rate compared with resting state [41]. In the current study, GXT increased HR significantly indicating greater sympathetic activity, and possibly activating sIgA transcytosis in the cells of the submandibular glands as has been reported in rodents. This condition could explain partially the higher [sIgA] after the GXT. Willemsen et al., (2000) suggested this mechanism [42]. Indeed, our participants engaged in strenuous exercise of very short duration. As such, sympathetic stimulation may have only been of short-lived influence as heart rate and [sIgA] returned to basal levels 30 minutes after exercise. Therefore, it is possible that short term sympathetic activity is a good stimulus to improve respiratory immunity in obese men and is supported by animal-based, mechanistic research [39]. It is worth noting that the effect of GXT was specific to [sIgA] because total protein levels were not affected by the exercise. Others report similar findings in non-obese males [19]. These data are consistent with the notion that high-intensity exercise of short duration enhances, rather than suppresses, respiratory immune function in healthy individuals [43]. However, this is novel finding in obese men.
5. Conclusions
- In conclusion, a GXT performed on a cycle ergometer until exhaustion seems to have a specific effect on sIgA, since the total protein concentration in saliva did not significantly change in the salivary flow rate in physically active obese men. Additionally, these findings agree with other reports showing that short, strenuous exercise stimulates [sIgA] in non-obese men.
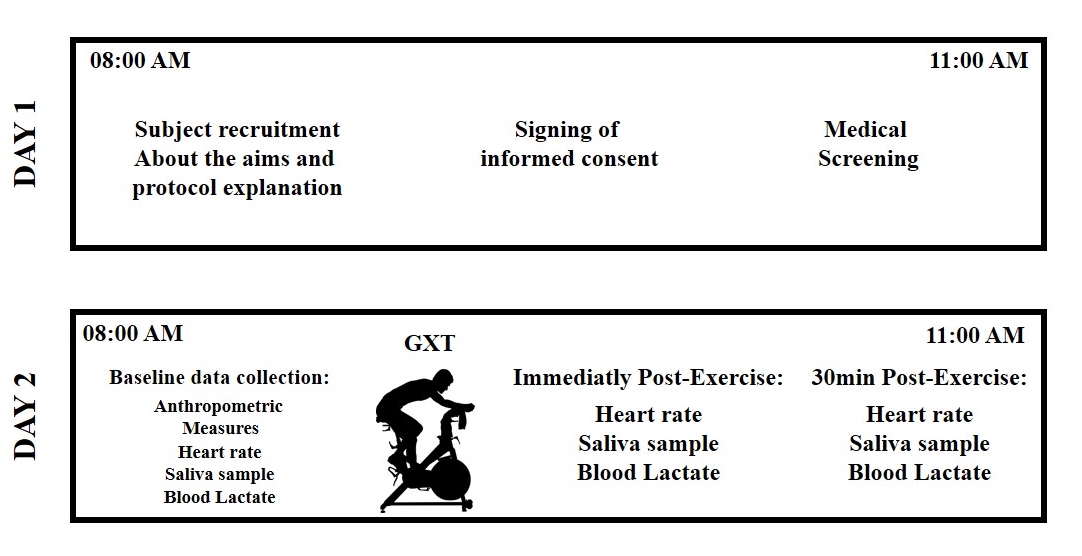 | Supplementary Figure. Design of the experimental procedures |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML