-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Sports Science
p-ISSN: 2169-8759 e-ISSN: 2169-8791
2018; 8(1): 25-37
doi:10.5923/j.sports.20180801.05

The Impact of Dietary Sodium Intake on Sweat Sodium Concentration in Response to Endurance Exercise: A Systematic Review
Alan J. McCubbin, Ricardo JS Costa
Department of Nutrition, Dietetics and Food, Monash University, Notting Hill, Victoria, Australia
Correspondence to: Alan J. McCubbin, Department of Nutrition, Dietetics and Food, Monash University, Notting Hill, Victoria, Australia.
| Email: |  |
Copyright © 2018 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The collection, processing, and analysis of sweat samples to determine sodium losses during endurance exercise is common amongst sports and exercise nutrition practitioners, and necessary for researchers investigating sodium losses and replacement strategies. Several factors influence sweat sodium concentration ([Na+]) that need to be controlled or considered when interpreting results. Dietary sodium intake in the days preceding exercise is one factor that may influence sweat [Na+]. A systematic review was undertaking using six databases (CINAHL, Embase, Medline Ovid, Scopus, SPORTDiscus, and Web of Science) to determine the impact of dietary sodium intake on sweat [Na+] in response to endurance exercise. Six papers met the inclusion criteria. They varied in the level of sodium intake (<196 to 9177 mg/d), intervention timeframe (3 to 42 days), exercise modality (cycling ergometry, treadmill walking and running), and sweat collection method (whole body washdown and regional patch techniques). Two studies showed significant differences in sweat [Na+] due to diet, two showed no significant difference, and two were not analysed statistically. No relationship was found across studies comparing the difference in sodium intake between interventions and sweat [Na+]. Several limitations were identified, including lack of validation of the intervention, collecting regional sweat samples from limited sites or averaging results across sites or collection days, and lack of statistical analysis. It is concluded that the impact of dietary sodium intake on sweat [Na+] in response to endurance exercise remains uncertain, however the review provides useful insights into the optimal study design for future research in this area.
Keywords: Salt, Dietary sodium, Sweat sodium, Physical activity, Endurance exercise
Cite this paper: Alan J. McCubbin, Ricardo JS Costa, The Impact of Dietary Sodium Intake on Sweat Sodium Concentration in Response to Endurance Exercise: A Systematic Review, International Journal of Sports Science, Vol. 8 No. 1, 2018, pp. 25-37. doi: 10.5923/j.sports.20180801.05.
Article Outline
1. Introduction
- During exercise, thermoregulatory strain increases sweat production to promote evaporative cooling and subsequently homeostatic thermoregulation [1]. The composition of sweat includes the electrolyte sodium, in typical concentrations ranging from 12 to 105 mmol/L [2]. Current sports nutrition guidelines recommend replacement of sodium during exercise “…when large sweat losses occur…”, although no specific values are currently suggested [3]. It is common practice for sport and exercise professionals working in the nutrition field to request or perform the collection, processing, and analysis of sweat samples to determine exercise-induced sodium losses, anecdotally for the purpose of planning sodium replacement strategies for athletes during training and competition. This suggests analysis of sweat sodium concentration ([Na+]) is highly sought after by practitioners, athletes and their support crews, with the belief that such testing will provide useful information that optimises nutrition strategies and subsequently improves athlete health and/or performance. Therefore, accurate and consistent measurement of sodium losses during exercise is of considerable importance to both researchers undertaking studies of sodium replacement, health and performance in athletes, and for athletes and their support crews wishing to establish sodium losses in competition from samples obtained during training. Several factors are known to influence sweat [Na+] during exercise, and therefore must be considered when testing athletes or designing studies in this area. The initial precursor sweat that is produced by the gland’s secretory coil is generally similar in composition to plasma [4]. For this reason, fluid balance will influence precursor sweat, and subsequently the composition of the excreted sweat. It has been shown that an exercise-induced body mass loss of 2.4% (i.e., mild dehydration) resulted in an increased sweat [Na+] compared to complete fluid replacement (91 vs. 81 mmol/L) [5]. The reabsorption of sodium that occurs as precursor sweat travels along the sweat duct is influenced by both the flow rate through the duct, and function of the gland itself [4, 6]. The flow rate of sweat is determined by its rate of production [7]. This in turn is influenced by several factors during exercise, including exercise intensity, ambient conditions, and airflow over the skin [1, 8, 9]. The reabsorptive function of sweat glands also appears to be somewhat adaptive to homeostatic mechanisms. For example, the effect of heat acclimation on sweat [Na+] is well documented, whereby ten days of acclimation for 100 min at 45°C lowered sweat [Na+] from 55 to 36 mmol/L, despite an increase in sweat rate which would usually be expected to have the opposite effect [10]. Therefore, it is clear that each of these factors must be carefully controlled during sweat collection, or at least factored into interpretation of sweat [Na+], when applying the results from one sweat collection to another exercise bout.Similar to heat acclimation, it has been suggested that sodium intake in the days prior to an exercise bout may also influence sweat [Na+], through altered sweat gland function [11, 12]. Early studies in this area were published just prior to, and following the Second World War [13-17]. In general, these studies indicated significant reductions in sweat [Na+] when sodium intake was heavily restricted. However, most of these studies measured sweat [Na+] at rest and exercise, often at intensities lower than expected for athletes during training or competition, in sedentary or untrained populations, and using significant sodium restrictions designed to create deficiency. Given that the sweat rate and sweat [Na+] is likely to be low in such conditions, any sodium deficit incurred in a single exercise bout is likely to be small. In addition, severe sodium restriction amongst active individuals would be extremely unlikely to occur in practice. It is therefore unclear how relevant these early findings are to current day athletes. Considering the known factors that regulate sweat sodium losses, and previous research identifying a dietary link to sweat [Na+], the intake of dietary sodium in the days preceding endurance exercise may be an important variable to control when collecting sweat samples from individuals partaking in physical exertion, either for research purposes or to inform practice. With this in mind, the aim of the current systematic review was to determine the impact of dietary sodium intake on the sweat [Na+] in response to endurance exercise.
2. Methods
- A systematic literature search was undertaken by two researchers, to determine the impact of dietary sodium intake in the preceding days on the sweat [Na+] during endurance exercise in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) statement [18].
2.1. Search Strategy
- A three-step search was undertaken of published English-language studies in six online scientific databases from inception to December 2017 (CINAHL, Embase, Ovid MEDLINE, Scopus, SPORTDiscus, and Web of Science). In addition, the reference lists of all identified studies and other known review papers relevant to the topic were searched to identify additional studies that may have been missed by the original search. In order to obtain the level of methodological detail required, book chapters, opinion articles, reviews, unpublished works, abstracts, short reports, and case-studies were not considered. The keywords applied in the literature search are shown in Table 1.
 | Table 1. Search strategy for the systematic review on the impact of dietary sodium intake on sweat sodium concentration in response to endurance exercise |
2.2. Eligibility Criteria
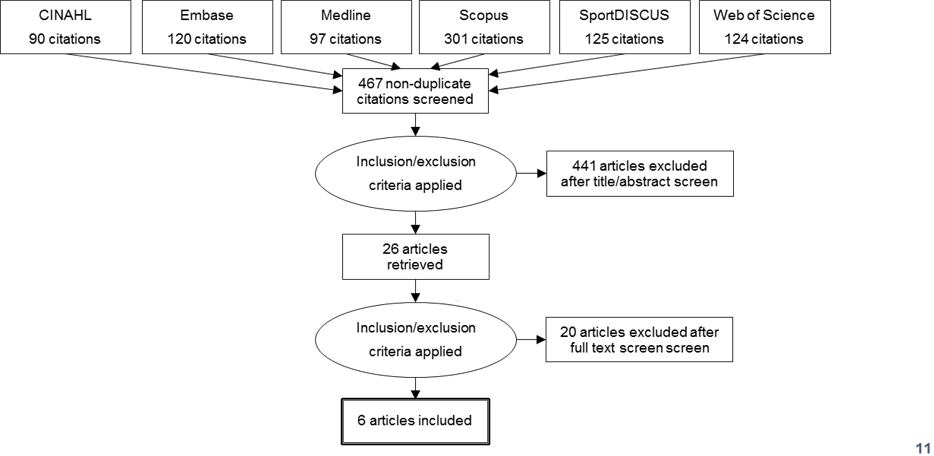
- Eligibility criteria were established by the researchers a priori in accordance with the Participant-Intervention- Comparator-Outcomes-Study (PICOS) design format [18]. Original field observational studies and/or laboratory controlled trials, presenting quantified data on sweat [Na+] from samples obtained in a healthy (i.e., absence of illness and disease) adult human population that required participants to perform an acute endurance exercise bout were considered for the review. Studies were suitable for inclusion if they involved either an observation of sodium intake that was quantified using food records and/or 24 hour urinary sodium excretion, or a prescribed sodium intake that was validated using the same methods, for at least twenty-four hours prior to the exercise bout. After duplicates were removed, the titles and abstracts were reviewed by two researchers against the eligibility criteria (Figure 1).
 | Figure 1. PRISMA diagram, showing the inclusion and exclusion of papers in the review |
2.3. Data Extraction
- Extraction of relevant data was then performed by one researcher and cross-checked by a second researcher. Variables extracted were the number of participants, their age, training status (including VO2max where available) and heat acclimation status; dietary intervention (sodium intake either provided or assessed by 24h urinary Na excretion); exercise protocol used; environmental conditions during the exercise bout; sweat sampling technique; pre-exercise hydration status and sweat losses during the exercise bout; and sweat [Na+], with means reported where available to examine differences. During the data extraction process, eligibility was again checked, and appropriate inclusion or exclusion action was taken. Any difference of opinion between researchers during the review process was resolved by discussion and consensus. Where possible, units were standardised by simple mathematical conversion. Where whole body sweat rate was reported as ml/m2/h, units were converted to L/h by calculating body surface area of participants from the mean participant characteristics, where available, using the equation of Du Bois and Du Bois [19]. Data were not considered appropriate for further synthesis into a meta-analysis due to the absence of homogeneous interventions and outcome measures.
2.4. Risk of Bias Assessment
- Risk of bias assessment was performed using the Cochrane ‘Risk of bias’ assessment tool [20]. The tool assesses the risk of selection bias (due to random sequence generation and concealment of allocation), performance bias (from inadequate participant blinding), detection bias (inadequate blinding of personnel conducting the study and those performing the outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective reporting of outcomes), and other potential forms of bias.
3. Results
3.1. Search Results
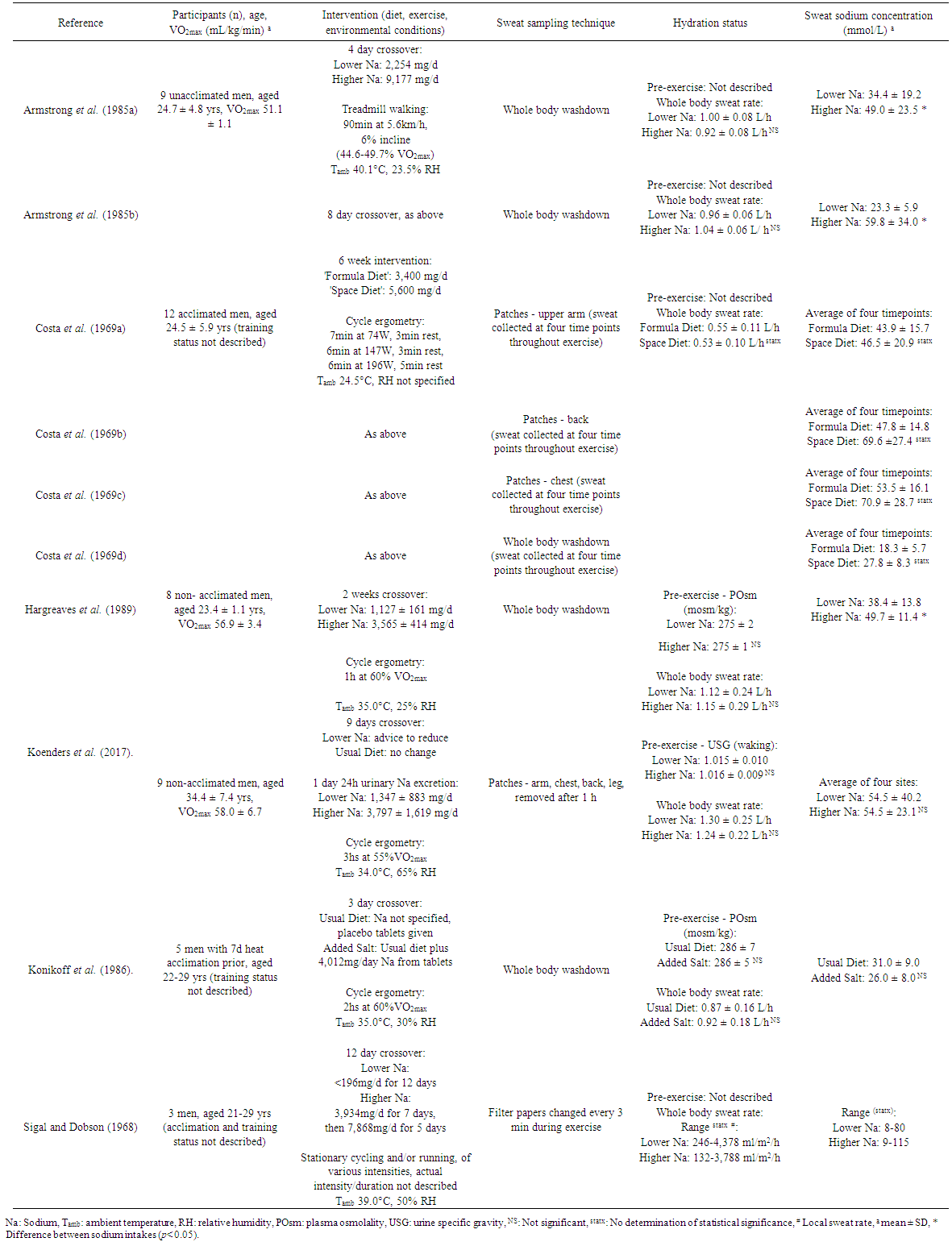
- The initial database search yielded 458 non-duplicate citations. 435 of these were excluded on title and abstract screening, leaving 23 papers. An additional two papers were included prior to full text review, based on the search of reference lists of review papers. Following the full text screening, six papers met all inclusion criteria and are included in this review (Table 2). Armstrong et al. [21] included sweat collection after four and eight days of dietary intervention, and is therefore presented as two parts. The paper of Costa et al. [22] included sweat collection data from three body sites, as well as whole body washdown, and is presented as four parts. A description and summary of each study’s protocol and results is presented in Table 2.
 | Table 2. Systematic review search results, showing included papers to determine the impact of dietary sodium intake on sweat sodium concentration when sweat samples were obtained during exercise only |
3.2. Study Characteristics
- Participants in all the included studies were male. Participant age varied between studies; two studies reported age as a range (21-29); the remaining studies reporting mean age which ranged between 23 to 34 years. Training status of participants was described using VO2max and was similar in Armstrong et al. (51.1 ± 1.1 mL/kg/min) [21], Hargreaves et al. (56.9 ± 3.4 mL/kg/min) [23], and Koenders et al. (58.0 ± 6.7 mL/kg/min) [24], but was neither quantified nor described in the remaining three studies.Four of the studies utilised cycling ergometry as the exercise modality. Three used steady state cycling at an intensity of 55-60% VO2max, with durations varying from 1 to 3 h (22-24), although in the three-hour study sweat samples were only collected in the first hour [24]. Costa et al. [22] utilised a protocol of three increasing intensities, interspersed with rest periods for a total of 40 min. Armstrong et al. [21] utilised 90 min of treadmill walking, whilst the study of Sigal and Dobson [26] involved a combination of stationary running and cycling, the duration and intensity of which is not described. Except for Costa et al. [22], which was performed at an ambient temperature of 25°C, all other studies were performed in temperatures ranging from 34 to 40°C. Of these, all studies other than that of Koenders et al. [24] were performed with a relative humidity of 50% or below.Pre-exercise hydration status was described in only three of the six studies, using either plasma osmolality [23, 25] or urine specific gravity [24]. In the three studies that did report pre-exercise hydration markers, none were significantly different between interventions, and all were in the range that would be considered euhydrated [1]. Because of the large variations in exercise intensity, duration and ambient conditions, the reported sweat rates varied markedly between studies, ranging from 0.53 to 1.30 L/h. This corresponded to a reduction in body mass between 0.7 to 1.6%. Importantly, within study sweat rates were not statistically significantly different between dietary intervention groups. Post exercise hydration status was only reported in one study, and due to the exercise protocol (three hours cycling, but sweat collection only performed in the first hour), the data cannot be interpreted in relation to the composition of the sweat samples [24].Sweat collection method also varied between studies, with the whole body washdown (WBW) method [21, 23], and regional patch method using either absorbent patches or filter papers [24, 26] used across all studies. Costa et al. [22] used both of the above methods, as well as collection from a sealed arm bag, however the arm bag data has been excluded from this review due to the established interference of this technique on sweat gland function and subsequent sweat composition [27].
3.3. Dietary Intervention
- Dietary sodium intake interventions varied greatly between studies. The mean sodium intake across studies could not be calculated due to differences in data reporting, but the range for the lower sodium intervention was <196 to 3400 mg/d, and 3565 to 9177 mg/d for the higher sodium intervention. The mean difference between interventions was calculated from either planned intake, actual intake (where available), or 24 hour urinary sodium excretion, and was 4305 mg/d across studies. The largest difference in sodium intake between interventions was 6923 mg/d [21], whilst the smallest difference was 2439 mg/d [23]. In all but one study, a prescribed diet was provided to participants. However, only two studies attempted to blind participants to dietary sodium intake, providing the identical diet plus either sodium or placebo capsules [23, 25]. The study of Koenders et al. [24] did not prescribe or provide a specific sodium intake; instead participants were counselled to consume a higher or lower sodium diet, and intake was estimated from a single day 24 h urinary sodium excretion on the final day of the intervention.
3.4. Effect of Diet on Sweat [Na+]
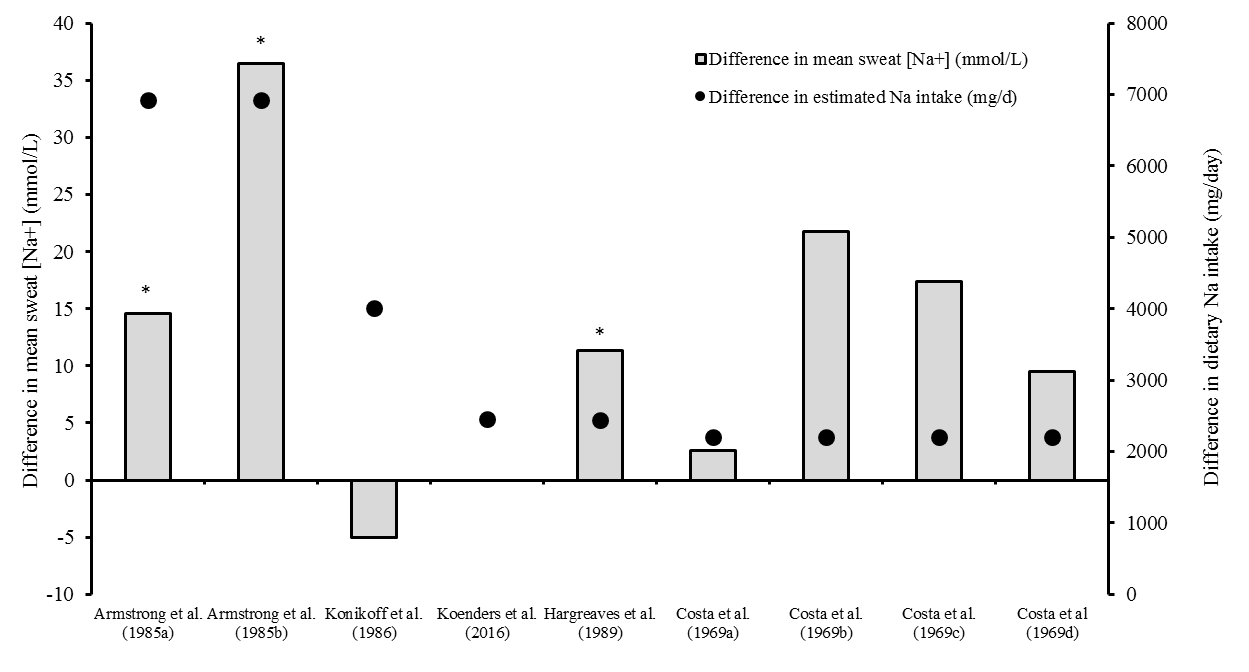
- Of the six studies included in this review, two found a statistically significant difference in sweat [Na+] attributable to changes in dietary sodium intake [21, 23]. In contrast, the studies of Koenders et al. [24] and Konikoff et al. [25] did not show a statistically different sweat [Na+] between dietary interventions. The study of Costa et al. [22] was not analysed to determine statistical significance. Whilst summary statistics were provided, the data distribution could not be determined, therefore no further statistical analysis could take place. Regardless, post-hoc power calculations suggest that this study was grossly underpowered to detect changes in sweat [Na+] whether parametric or non-parametric tests were used (1-β if parametric: arm 0.06, back 0.34, chest 0.22, WBW 0.56; if non-parametric: arm 0.06, back 0.32, chest 0.21, WBW 0.54), because participants in each intervention group were independent of each other, rather than the crossover design used in all other studies. Sigal and Dobson [26] did not report mean sweat [Na+] data; instead, sweat was collected every 3 min during exercise, and data presented as ranges for each individual throughout the exercise bout, for both levels of sodium intake. The lower end of the range was similar between diets, but the higher end of the range was between 17-71 mmol/L greater from days three to five with the higher sodium intake in every participant. The effect of sodium intake on the magnitude of change in sweat [Na+] was also examined. Figure 2 shows no relationship between the difference in mean sodium intake between interventions and the subsequent change in sweat [Na+]. For studies where adequate dietary intake and sweat sodium data were available, sweat [Na+] increased between 0.0 to 0.99 mmol/L for every 100mg increase in daily sodium intake.
3.5. Risk of Bias Assessment
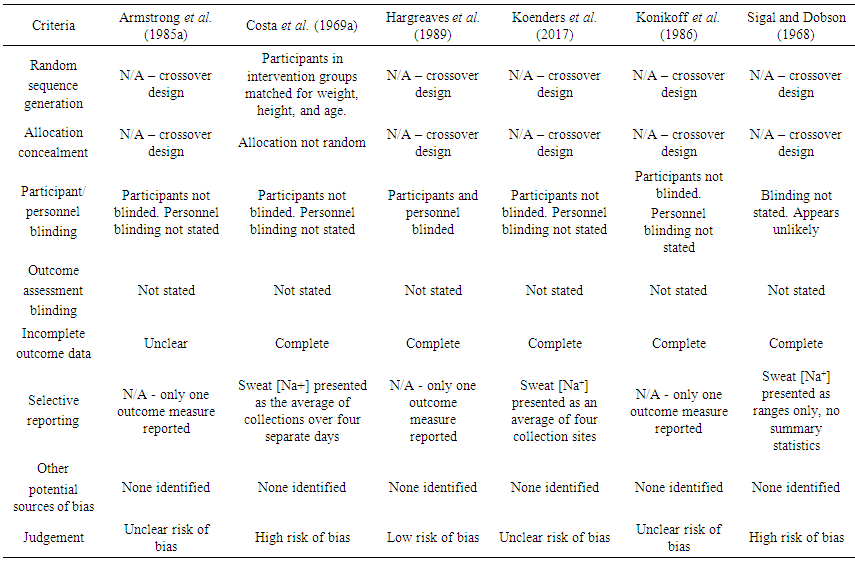
- Results of the risk of bias assessment are shown in Table 3. Selection bias was evident in the study of Costa et al., with participants “distributed between two dietary treatments to equate as nearly as possible the weight, height, and age of the two groups” [22]. Potential performance and detection bias was observed in five of the six papers, with only one documenting a double-blinded methodology in regards to the dietary intervention [23]. Reporting bias was seen in two studies, with averaging of results obtained across multiple sweat collection sites or time points [22, 24], and presentation of sweat [Na+] as a range only, with no additional summary statistics or test of significance [26]. Overall one paper was deemed low risk of bias [23], with three deemed unclear risk of bias [21, 24-25] and two high risk of bias [22, 26].
4. Discussion
- Sports nutrition practitioners commonly collect and analyse sweat samples of athletes, using this data to inform sodium replacement strategies. Researchers also collect sweat samples for research that investigates sodium replacement and exercise performance. The aim of the current systematic review was to determine the impact of dietary sodium intake on the sweat [Na+] in response to endurance exercise. The suggestion that usual dietary sodium intake influences sweat [Na+] has been reported prior to the Second World War [17]. From a sport and exercise nutrition perspective, however, there are only a few studies that examined sweat losses solely during a form of exercise that replicates training undertaken by recreationally active individuals, or athletes during competition. Only six papers were identified that met the inclusion criteria and were included in this systematic review, and, possibly due to the large differences in study designs, contrasting results were found. The findings from the six included studies were mixed; with statistically significant effects of diet found in two studies, no effect in two studies, and no statistical analysis performed in the remaining two studies.There are several factors that could contribute to the different findings between studies, including differences in the training status of the participants, sweat collection techniques and data reporting, the method of dietary intervention and control, and control of pre- and during exercise hydration status.
4.1. Study Methodologies
- Training status was described in only three of the six included studies. One described participants as sedentary, one recreationally active, and one highly trained, however the VO2max values across these studies differed by only 6.9 mL/kg/min. Whilst effect of training status on sweat rate is well documented, with greater sweat rates seen in trained compared to untrained populations [28-31], the effect on sweat [Na+] has received minimal attention [12, 28]. Given that there are examples of increased sweat rate causing an increase (with exercise intensity) and decrease (with heat acclimation) in sweat [Na+], it is clear that more research is required to better understand whether training status is important when comparing sweat [Na+] responses across different population groups. Therefore, studies investigating sweat [Na+] in the context of exercise should study populations of relevance to the study’s applicability (i.e., athletes rather than sedentary individuals in the context of sport and exercise nutrition).Methods to determine exercise-induced sodium losses involve the collection, processing, and analysis of sweat samples, and either direct (e.g., complete sweat collection) or indirect (e.g., body mass changes) measurement of sweat water losses [1, 27]. Whole body washdown is considered the reference method for sweat collection as it directly collects all sweat produced, and does not disturb usual sweat secretion or evaporation from the skin [32]. However, this technique is limited to stationary cycling in a laboratory setting, cannot realistically simulate outdoor airflow or humidity, and often involves the participant exercising with unfamiliar clothing and equipment [27, 32]. Ventilated capsules and regional patch techniques, provided patches are removed before complete saturation, are also considered valid methods of sweat sample collection; albeit with an appreciation that sweat collected from individual sites does not represent the composition of whole body sweat [32, 33]. Of the six studies included in this systematic review, three utilised the whole body washdown method, with the remainder employing some form of regional patch technique (i.e., either absorbent patches or filter papers), or a combination of both. The consistency of methods used within each study allows for adequate comparisons of the change in sweat [Na+] between dietary interventions, and the sweat [Na+] found in all studies, regardless of collection method, fell within the ranges expected by healthy individuals during exercise [2].Another important consideration when interpreting studies using regional sweat collections, is that sweat gland function varies across different body regions [33]. Whilst the effect of dietary sodium intake on sweat [Na+] across different body sites is not currently known, it has been shown that changes in sweat gland function to environmental interventions do not occur consistently across all body sites [34]. Therefore studies utilising patches at only one site may potentially either miss important changes occurring elsewhere, or overestimate the degree of change relative to the whole body. In addition, the way that data from multiple regions is reported is also important. Koenders et al. [24] reported the average of sweat [Na+] collected from patches simultaneously at four sites, but did not account for the proportional representation of each site to the whole body surface area, a method that has been validated against whole body washdown in the past [32]. Costa et al. [22] collected sweat samples at four time points over a six week period, reporting the average. Both approaches may have misrepresented the findings from each site at each time point of sweat collection. Future studies in this area should use either whole body washdown or regional sweat collection techniques collected across several body sites, reporting the data from each site separately or proportionally weighted rather than averaged. The majority of included studies provided food to participants in order to carefully control sodium intake, albeit most did not explain how compliance with the prescribed diet was assessed, either by participant reporting or validation by means of 24 h urinary sodium excretion. Urinary sodium excretion has long been considered the reference method for estimating dietary sodium intake, in both individuals and populations [35]. However, a recent study that tightly controlled sodium intake for several weeks showed significant fluctuations in daily urinary sodium excretion, despite no change in sodium intake [36]. This study found that a single day 24 h urinary sodium excretion would incorrectly identify differences in sodium intake of 1200 mg/day about half of the time. Increasing the number of consecutive days of urine collection (i.e., three days) improved the accuracy to 75%, with seven consecutive days accurate in 92% of cases [36]. Four of the six papers identified in this review reported none or only one day of urinary sodium excretion data, and either lacked records of actual sodium consumption from a prescribed diet or did not provide a specific sodium intake to all participants, and therefore should be interpreted with caution [22, 24-26]. It should also be noted that in three studies participants were regularly exercising in the heat throughout the dietary intervention [21, 25, 26]. Because of the inevitable sweat sodium losses, which may be greater during the high sodium dietary intervention, the difference in sodium balance between interventions is likely to have been less than the data suggests, due to increased overall sodium losses from both sweat and urinary excretion [37]. This is particularly true when interpreting urinary sodium excretion data, as significant sweat sodium losses are likely to offset urinary losses [37, 38]. Participants were reported to have refrained from exercise for only one day, and only in one study [24], also suggesting that the effect of the high sodium intake may have been less than intended. Two studies did not report physical activity in the days preceding sweat collection, preventing any interpretation of its potential impact on sodium balance [22, 23]. Differences between dietary interventions in pre-exercise hydration status or fluid intake during the exercise protocol could also theoretically contribute to the contrasting results between studies. Because precursor sweat composition generally mimics that of the surrounding interstitial fluid, and therefore plasma electrolyte composition [4], changes in pre- and during exercise hydration status will likely be reflected in both precursor and final sweat composition [5, 6, 39]. Because of this, the control and confirmation of pre- and during exercise hydration status is an essential part of studies in this area. Well accepted measures of pre-exercise hydration status include total body water, plasma osmolality, and waking urine specific gravity [40]. Whilst most studies reported pre-exercise plasma sodium, only three of the included studies attempted to measure any of the above variables [23-25].
4.2. Mechanisms for Altered Sweat Gland Function in Response to Dietary Sodium Intake
- The finding of altered sweat [Na+] in response to changes in dietary sodium intake in some studies prompts the question as to the mechanism by which this could occur. Since precursor sweat generally mimics plasma electrolyte composition [4], it seems more likely that alterations in excreted sweat composition are due to changes in the degree of sodium reabsorption by the absorptive duct of the sweat gland. Only one paper included in the current systematic review attempted to estimate the rate of sweat secretion and/or reabsorption by the sweat gland mathematically [26]. In this study, water-free sodium reabsorption was 15 to 47% greater at all time points and in all participants during the low sodium diet, supporting the hypothesis for increased sodium conservation in the face of lowered dietary sodium intake. The exact mechanism by which sweat sodium reabsorption may be regulated in response to dietary sodium intake is not completely understood, although plausible mechanisms have been proposed. Reabsorption of sodium from sweat occurs in two parts. Firstly, absorption of sodium into endothelial cells that line the sweat duct occurs through the endothelial sodium channel (ENaC), and chloride through the cystic fibrosis transmembrane conductance regulator (CFTR), and their rates of reabsorption are tightly linked [6, 41]. Sodium is then actively transported from the cell, across the basal membrane and into the interstitial space by the action of Na+/K+-ATPase transporters. Plasma aldosterone increases with reduced sodium intake, and has been associated with increased sodium reabsorption in the distal sweat gland, and lower sweat [Na+] in excreted sweat [4, 42]. Two of the studies included in this review reported plasma aldosterone [23, 25], whereby greater sodium intake resulted in significantly lower plasma aldosterone as expected. Grand et al. [43] administered aldosterone intravenously and intramuscularly before inducing sweating by pilocarpine iontophoresis in adults and children, and found a reduction in sweat [Na+] in both groups. Sato and Dobson [44] showed that intracutaneous injection of aldosterone reduced sweat [Na+] during exercise within 24 h of administration at two different sweat rates, although it should be noted that the same authors stated almost twenty years later that “it is not certain that aldosterone is the principle regulator of ductal absorption” [4], and we are unaware of any literature since then that has provided exogenous aldosterone systemically, and subsequently measured sweat [Na+], during exercise or otherwise. Although parallels are frequently made to aldosterone’s role in sodium reabsorption in the kidney, it is becoming clear that the regulation of ion transport in sweat ducts is both very different and much more complex than first thought [6, 45-47].One possible explanation that is yet to be investigated is the effect of osmotically inactive sodium storage, primarily in the skin [48]. There is evidence that increased dietary sodium intake results in greater osmotically inactive sodium storage in humans [49], and evidence that the amount of these stores play a role in regulating natriuresis, albeit in animal models [50]. To the best of our knowledge there are no studies to date that examine the relationship between osmotically inactive sodium storage and sweat gland function. Further mechanistic research to confirm a causal link between dietary sodium intake and sweat [Na+] is warranted.
4.3. Study Limitations
- One limitation to this systematic review is a potential for other existing literature to have been missed when utilising the search strategy described. This risk was minimised by searching the reference lists the identified papers, and of known review papers relevant to the topic. This allowed the inclusion of two additional papers, although both were subsequently excluded during the full text review. The other obvious limitation was the lack of meta-analysis. Given the heterogeneity in study designs, lack of data reported in some papers and the low likelihood of obtaining additional data given the age of most of the included papers, it was determined that meta-analysis would not be possible.
4.4. Future Study Design
- Because a potential role of diet in the preceding days on sweat sodium losses during exercise has significant implications for both researchers and practitioners, and the existing literature has found conflicting results and has significant risk of bias, further investigation of this question appears warranted. Existing studies in this area differ significantly in their methodology, with several potential limitations identified to each of them. Ideally, future studies should include: 1) the use of competitive athletes as study participants, as there may be important differences from sedentary or relatively untrained populations [12]; 2) use of a blinded sodium intake, with food and/or sodium capsules that are provided to participants to ensure the most accurate estimates of intake [51]; 3) intake verified with at least three consecutive days of 24 hr urinary sodium excretion data, and either abstinence or the capture of exercise-induced sweat sodium losses if exercising during this time [36, 38]; 4) exercise protocols which reflect real-world endurance training and/or competition, and their expected sweat rates [7, 8]; 5) control and accurate measurement of pre and post-exercise hydration status, accurate assessment of sweat water losses during exercise, and prescriptive fluid replacement to standardise hydration status between participants [5]; 6) careful control of ambient conditions, including temperature, humidity, and airflow [1, 9]; 7) use of a well validated sweat collection technique - WBW is the reference method but the regional patch method is easy to apply, well validated and translational to real-world settings [32]; 8) sampling sweat from multiple sites when using regional collection methods, and reporting the individual site data or proportionally calculated estimates of whole body sweat [Na+] [12, 32]; 10) use of well validated sweat sample analysis techniques [52], and; 11) measurement of biomarkers that provide insight into the mechanisms underlying any differences in sweat gland function in response to dietary sodium intake [7].
4.5. Implications for Research and Practice
- Current sports and exercise nutrition guidelines and recommendations encourage endurance athletes to reduce training volume and to increase carbohydrate intake for one to three days before competition, with the aim of increasing glycogen storage [3]. This conscious increase in food intake may, either deliberately or unknowingly, increase sodium intake significantly. In addition, anecdotal evidence suggests that many endurance athletes deliberately increase sodium intake in the days preceding competition, in the belief this will optimise pre-race hydration and sodium status, assisting the maintenance of exercise performance during competition. For these reasons, sweat sodium losses measured at one time point (i.e., during training) may not reflect losses expected at another (i.e., competition day), due to differences in sodium balance in the preceding days. Further research is required to confirm whether this is indeed the case, and to determine whether there is a need for strict standardisation of sodium intake for research studies that investigate differences in sweat sodium losses or sodium replacement strategies, and to allow practitioners to interpret sweat composition data in the context of sodium intake.The mean differences in sweat [Na+] seen between dietary interventions ranged from -5 to 36.5 mmol/L across studies identified in this systematic review. Using the midway point of this range (15.75 mmol/L) as an example, this data can be combined with sweat rate data obtained during endurance events to explore the potential implications of dietary sodium intake on sweat sodium losses during these events. Using this approach, one might anticipate a difference in total sweat sodium losses of 1360mg during a marathon raced by amateurs in 3:45:53 in thermoneutral conditions [41], or 1736mg during a marathon raced by world class runners finishing in a time of 2:06:31 in a hot and humid environment [53]. This represents a difference of 2-3% of total body sodium stores lost, given that total body sodium is approximately 1g/kg body mass [54]. In ultra-endurance events, the anticipated difference in hourly sweat sodium losses would be lower due to a lower relative exercise intensity of around 50-60% VO2max [55-57], and subsequently lower sweat rate. However, the significantly increased duration of these events results in higher overall sodium losses. During an Ironman triathlon held in thermoneutral but humid conditions [58] (Speedy et al., 2001), theoretical differences in sodium losses due to diet for recreational triathletes could be as high as 3507mg, or more than 5% of total body sodium stores, over a total race duration of 12:18:00. These differences could have profound implications whereby athletes fail to adequately replace sodium due to an underestimation of sweat losses, resulting from sweat testing during a period of lower sodium intake, whilst competing following a period of greater intake. Although there is currently minimal evidence that sodium replacement is important for endurance performance, potential health consequences of inadequate sodium replacement during prolonged exercise include increased risk of exercise-associated hyponatraemia [59], and small, acute losses of bone mineral content which may become cumulative over time [60].
5. Conclusions
- Several studies have indicated that dietary sodium intake influences sweat [Na+] in sedentary populations or over time periods spanning both rest and exercise. However, our systematic review found that this effect was unclear when including only studies that measured sweat [Na+] specifically during endurance exercise, possibly due to methodological differences of many of the included studies. Several limitations were identified with the included studies, including lack of validation of the intervention, collecting regional sweat samples from limited sites or averaging results across sites or collection days, and lack of statistical analysis. The review however provides useful insights into the optimal study design for future research in this area.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML