-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Sports Science
p-ISSN: 2169-8759 e-ISSN: 2169-8791
2018; 8(1): 1-7
doi:10.5923/j.sports.20180801.01

Blood Biochemical Markers of Competitive Bodybuilding Athletes Users of Anabolic Androgenic Steroids and/or Growth Hormone (AAS/GH), Strength Athletes Drugs Free and Sedentary Persons
Carlos Alexandre Fett1, 2, 3, Marcos Maruyama1, Camila Fernanda Costa e Cunha Moraes Brandão1, 2, Waléria Christiane Rezende Fett1, 2
1Nucleus of Research in Physical Fitness, Informatics, Metabolism, Sport and Health (NAFIMES), Faculty of Physical Education, Federal University of Mato Grosso, Cuiabá, Brazil
2Master Degree in Physical Education, Faculty of Physical Education, Federal University of Mato Grosso, Cuiabá, Brazil
3Doctored Degree Program of Faculty of Medical Science of Federal University of Mato Grosso, Cuiabá, Brazil
Correspondence to: Carlos Alexandre Fett, Nucleus of Research in Physical Fitness, Informatics, Metabolism, Sport and Health (NAFIMES), Faculty of Physical Education, Federal University of Mato Grosso, Cuiabá, Brazil.
| Email: |  |
Copyright © 2018 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Many professional athletes and those frequenting gyms use Anabolic Androgenic Steroids and/or Growth Hormone (AAS/GH), to improve performance and aesthetics. The aim of this study was to compare the blood markers of 03 groups with 08 volunteers in each: bodybuilders using AAS/GH (A), amateur strength athletes drugs free (B) and sedentary individuals as control (C). The tests Kruskall-Wallis (ANOVA Non-Parametric) and followed by the Dunn was carried out (p<0,05). There was a significant increase in Group A in comparison with B and C to: GH, lactate dehydrogenase, urea, aspartate aminotransferase, alanine aminotransferase, low-density-lipoprotein cholesterol (LDL), total cholesterol/high-density-lipoprotein cholesterol (HDL) and LDL/HDL ratios; and reduced levels of: sex hormone binding globulin, insulin, HDL. The creatine phosphokinase was increased in Groups A and B, and creatinine in A regarding C but equal B. The groups were equal for TT and free testosterone, cortisol, leptin, ALP, TC, TAG, VLDL-c, homocysteine and TT/cortisol ratio. These results suggest that users of AAS/GH were exposed to a higher systemic risk, but not so intense as expected. In this way, point out that AAS/GH user’s protocols, should be consider the interactivity of diet, training and genetic for the building the standard position to bodybuilding and strength athletes.
Keywords: Lipid profile, Renal function, Muscle enzymes, Hepatic enzymes
Cite this paper: Carlos Alexandre Fett, Marcos Maruyama, Camila Fernanda Costa e Cunha Moraes Brandão, Waléria Christiane Rezende Fett, Blood Biochemical Markers of Competitive Bodybuilding Athletes Users of Anabolic Androgenic Steroids and/or Growth Hormone (AAS/GH), Strength Athletes Drugs Free and Sedentary Persons, International Journal of Sports Science, Vol. 8 No. 1, 2018, pp. 1-7. doi: 10.5923/j.sports.20180801.01.
Article Outline
1. Introduction
- Anabolic-androgenic steroids (AAS) are synthetic derivatives of the hormone testosterone and it is usually administered orally or via injection and is by far the anti-doping rule violation most detected in the laboratories tests [1]. In the first half of the past century these drugs were used by medicine because up until now, they are effective for the treatment of hypogonadism and other chronic diseases, which present cachexia as the prognosis (e.g., chronic renal disease, chronic obstructive lung disease, cancer, Acquired Immune Deficiency Syndrome - AIDS), depressive disorders, etc. At a second stage the AAS and other drugs such as the growth hormone (GH), began to be used in the sports [2-4].Thus, usual resistance training practitioners, among them adolescents, have been observed making indiscriminate use of androgenic steroids and/or growth hormone (AAS/GH) for aesthetic purposes [2-6]. It is alarming that the large majority of these users not are counseled about the risks of these drugs, as there has few health professionals that have technical information about this situation [3]. About the effects of AAS/GH that have been established, we may point out the improvement in performance, increases in both muscle mass and strength, as well as considerable improvement in tolerance to physical exercise [7-9]. On the other hand, a well conduced double blind study that control the AAS intake 12 times during a month in athletes (n=9 placebo; n=8 testosterone undecanoate; n=8 19-norandrostrenedione) during a hard endurance training do not find difference in performance and physical stress level of the athletes after a maximum treadmill test [10]. As regards the negative effects of AAS on health, there are neuropsychiatric alterations, such as mood changes and aggressive behavior, anxiety, depression, paranoia, confusion, amnesia [1, 4, 11], hepatotoxicity [1, 11, 12], renal complications due to reduction in creatinine clearance, increase in blood pressure due to albuminuria, and morphological cardiac alterations, left ventricular hypertrophy, myocardial dysfunction [1, 11, 13], thromboembolism, intracardiac thrombosis, stroke, arrhythmias, cardiomyopathies and sudden death [11]. Furthermore, there is association with sarcomas, neoplasms, renal, hepatic and prostate tumors linked to the use of AAS [11]. Beyond that, the unwanted effects do not stop with the cessation of administration the AAS, through the cellular memory mechanism [1]. Recently was observed in a competitive bodybuilding a Stevens-Johnson syndrome (SJS) that is characterized by skin problems [13].On the other hand, there are studies that do not report harm and even show evidence of beneficial adaptations provided by the use of AAS concomitant with performing physical exercise. Among them are the improvement in cardiac function resulting from the cellular and morphological changes, increment in vascularization of the myocardium, attenuation of the deleterious effects on lipoproteins [14] and an increase in submaximal aerobic resistance [8]. In addition, exercise per se seems to exert a protective effect on the adverse consequences of the use of AAS [15].But, we need point out that the majority of the deleterious effects of AAS are reported in researches with animal experimentation, reduced samples, inadequate control groups and/or case studies, which limit the impact of the results [8, 12-19]. Equally, one study despite to observe that the bodybuilders users of AAS had an impairment of the ventricular strain, the standard systolic echo parameters were in the normality, showing that the increase in cardiovascular risk factor was discrete [20]. Thus, the potential effects of the use of different AAS/GH in athletes has not been completely elucidated [3].In regard GH, there a little evidence on the side effects [9]. By the other hand the ergogenic effect was demonstrate in some studies [1, 9, 21]. To date, there is no method for classifying GH as doping [1].Thus, the aim of the present study was investigate the behavior of blood biochemical markers of competitive bodybuilding using AAS/GH with others two groups, athletes drugs free and sedentary persons.
2. Methods
2.1. Human Volunteers and Eligibility
- Fifty competitive bodybuilding AAS/GH users were invited to participate of the present study, but just 08 answers positively and/or fulfill all required procedures after one year of followed. The n was established at eight for the other two groups, due to equalizing them to the number and similar profile of bodybuilders. The bodybuilding were national and international competitive athletes AAS/GH users (Group A, n=08); amateur strength athletes drugs free (Group B, n=08), and sedentary persons (Group C, n=08) (Figure 1).
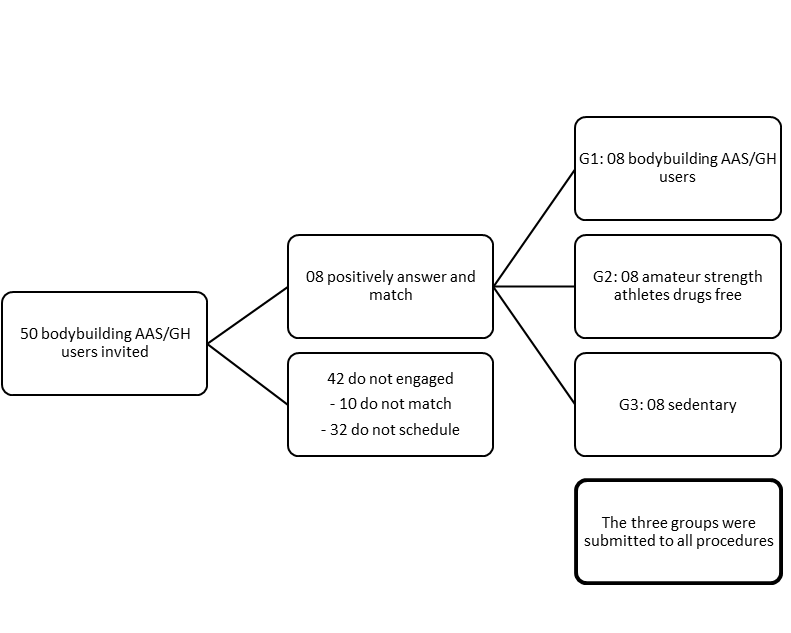 | Figure 1. Design of the study and split of the Groups |
2.2. Blood Collection
- Were performed by the "Carlos Chagas Laboratory" (Cuiabá-MT) after 12 hours fasting, and were always collected the day after performing resistance training considering Groups A and B. The methods used for analyses were as follows: total cholesterol and triacylglycerol (TG) by enzymatic/oxidase; lipoproteins (VLDL-c, LDL-c e HDL-c) by selective inhibition and calculation; urea and creatinine by urease and alkaline picrate, respectively; Creatine Phosphokinase (CPK) concentrations (Method UV – NADH; Creatine Kinase); aspartate aminotransferase (AST) (EC 2.6.1.2); alanine aminotransferase, (ALT) (EC 2.6.1.2); and alkaline phosphatase (ALP) (Kinetic P Method – Nitrophenol; kit alkaline phosphatase (ALP); all using Olympus® kits); total and free testosterone, GH, sex hormone binding globulin (SHBG), insulin, cortisol and homocysteine were analyzed by the chemiluminescence method with Architect® kits; testosterone, Immulite® 2000 (hGH), Immulite® 2000 (SHBG), ADVIA Centaur® insulin (IRI), ADVIA Centaur® cortisol (COR) and ADVIA Centaur® homocysteine (HCY), respectively; leptin was analyzed by Enzyme Immunoassay (Millipore® kit); lactate dehydrogenase enzyme activity (LDH) by the lactate-pyruvate method (Lactic Dehydrogenase (LD; Olympus® kit).
2.3. Ratios
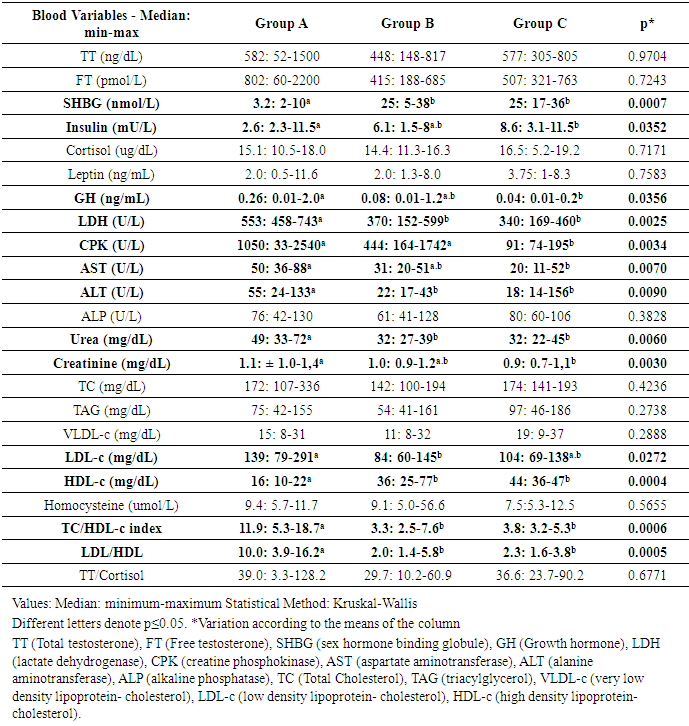
- Some ratios were calculated to refine the risk factors: Total cholesterol/HDL and LDL/HDL are used to predict ischemic heart disease risk [22], and; TT/cortisol ratio related to stress and training recover [10].
2.4. Drug Questionnaire
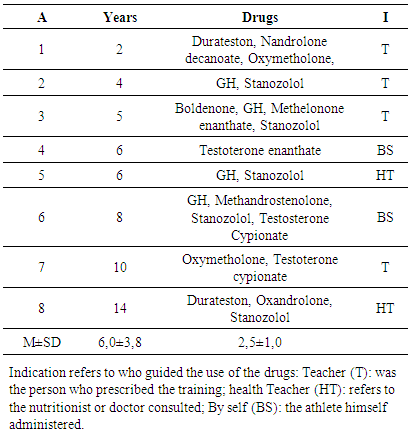
- All voluntaries of the Group A were answered the following questionnaire: 1. What drugs are you using? How long time? Who prescribe this?
2.5. Statistical Analyses
- Was used the Kruskall-Wallis (ANOVA Non-Parametric) test, followed by the Dunn multiple comparisons test to detect differences between the groups. To consider the testosterone total and free with SHBG and insulin the three groups were treated how just one, and correlated the variables one each other with multiple regression. The level of significance was p≤0.05 and the interval of confidence 95%.
3. Results
- The profile of the volunteers is shown in Figure 2, in which only the BMI was significantly more elevated for Group A.
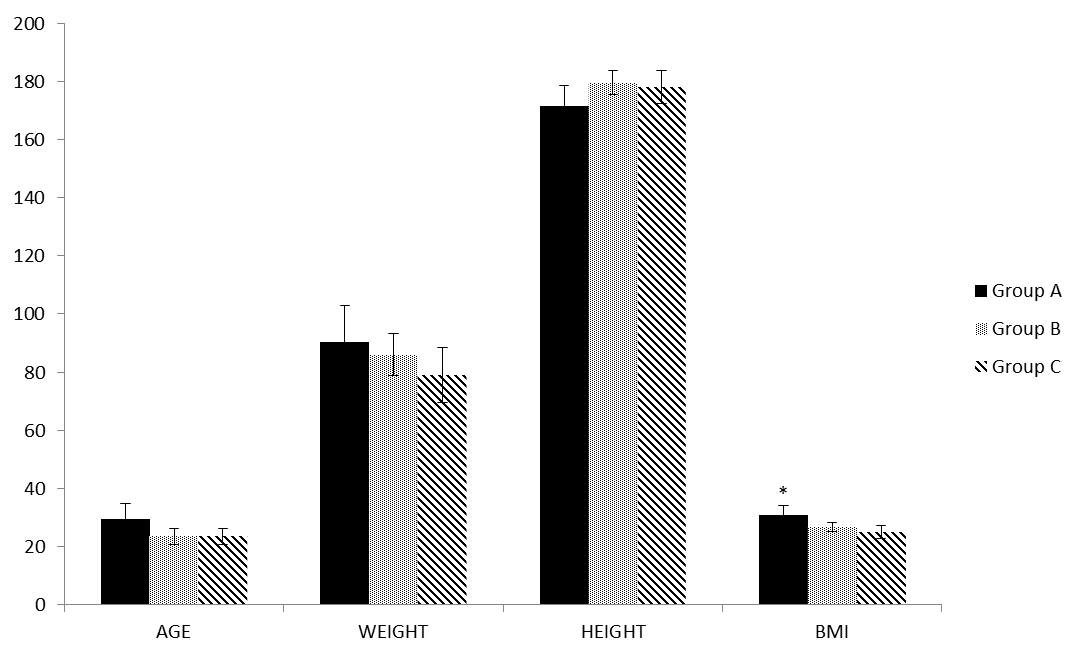 | Figure 2. Profile of voluntaries. They were similar with different significance just to Body Mass Index (BMI, kg/m2; p=0.0098) |
|
4. Discussion
- The profiles of the groups here studied were homogeneous, with the exception of BMI, which was higher in the bodybuilding (Group A), how expected [20]. Half of the Group A sample used 03 or more drugs, and the vast majority was prescribed by the teacher, different what was observed in another study that from 15, only one subject combined two AAS [24]. This denotes the importance of clearing policies among athletes and in the gyms, where young people have used these drugs indiscriminately [2, 3, 6].In general, the blood biochemistry was worse for Group A, that are in agreement with others [1, 12, 13], but this did not occur in a manner as intense as it did in these studies. It may be that some protective effect of physical exercise was acting, or even that the degree of toxicity of these drugs was not as intense as expected. This situation also having been observed in other studies [8, 15, 17, 18].Reinforcing this rationale, the physical exercise per se should be had a protective effects in the AAS/GH user, similar that observed in a study with stanozolol-treated rats in that the physical exercise neutralize the unwanted effects of the drugs [15]. In this regard, in the present study the stanozolol was the drug more consumed by the athletes. Different this, Souza and colleagues saw that just one subject of 15 from a group of AAS users consumed stanozolol and 11 they made use methyldrostanolone. The nandrolone decanoate and durateston were also consumed by one individual each [24]. Others found that in bodybuilders researched, the subjects used a variety of oral and parenteral AAS, in a cyclic fashion, typically spending 6–10 weeks on cycle, followed by 4–8 weeks off cycle [25]. These evidences that the variations in consumption should be the rule, not the exception, including a tough challenge to researcher these groups of athletes.Cortisol, total and free testosterone did not differ between groups, but, the SHBG a globulin attached to circulating testosterone together with albumin, was significantly lower in Group A. These results are controversial in literature with serum testosterone increases in AAS users [26], but observed by others a significant reduction in total testosterone [7, 10] and SHBG in AAS users [18] compared to non-users. We have found a great variability in the Group A and is possible that the use of AAS in a certain phase of the cycle alters the endogenous production of testosterone and SHBG, what was concluded in this regard in a systematic review [3].This reasoning is reinforced by the amplitude of the total and free testosterone levels within Group A in which there were three individuals with low levels (52 to 74 ng/dL; and 60 to 100 pmol/L) and three with high levels (1382 to 1500 ng/dL; and 1970 to 2200 pmol/L) respectively. Total and free testosterone were well correlated one each other but they were not significantly correlated to SHBG; in this respect differently to another study that find a good correlation of SHBG and total testosterone but, like us, no correlation of SHBG and free testosterone and a good correlation between testosterones total and free [27]. This sounds that the mechanisms involved were influenced by discrepant factors, like different kind of drugs and individuality.The other reasoning is that the physical exercise per se increases the level of the testosterone free and total, [28] cortisol and GH [29], what confound just the ergogenic aid. With reference to the high concentrations of GH, significant difference was shown only when Group A was compared with Group C. Furthermore, in Group A, four athletes were making use of exogenous GH. In this sense, one of the bodybuilding presented a very low value and one of the Group B was the second highest value of all for the GH. It is known that the high intensity of training as well as high levels of total and free testosterone are inversely correlated with the level of fasting insulin [27]. The fasting insulinemia was shown to be lower in Group A, with significance only with regard to Group C and different from that observed in the aforementioned study, showed no association with total and free testosterones. However, the situation observed here, suggests that the AAS/GH had a negative impact on insulin, in addition to the regulation caused by high intensity exercise. No athlete declared making use of exogenous insulin.The cortisol, TT/cortisol ratio and leptin levels did not differ among the groups. The increase in cortisol production may be attenuated by the use of AAS, even during bout of intense training [7]; but this is not linear which other study found the reduction in cortisol in placebo and 19-norandrostenedione groups but not in testosterone undecanoate after a maximal treadmill test. Yet, different of us, this study found that TT/cortisol ratio increase significantly in placebo but not in the other two groups [10]. In regard leptin, the consumption of AAS was associated with its decreases [25], condition that was not observed by us.Intense training promotes muscular micro injuries, which increase the CPK [7, 10] and LDH enzymes [30], which apparently occurred in the groups that trained, however, not systematically. The LDH level was significantly higher in Group A compared with the other two groups, but they did not differ between Groups B and C. CPK was higher in Groups A and B compared with Group C, but although it has statistical difference just between A and C, it is importantly increased in Group A. Another studies found no difference in this enzyme in bodybuilding [31] and endurance athletes [10] users and non-user of AAS. Contrary this, AAS-users increase less than non-user and return to basal levels in 24 h while non-users remained elevated in CPK levels after a bout of intense resisted exercise [7].Even though those not all cardiac risk factors altered in our study, some important variables were impaired in Group A. The reduced levels of SHBG [27], HDL-c [11, 18], and increased levels of LDL-c [11], TC/HDL and LDL/HDL ratios (2.5 and 2.6 x above normality respectively; [22]) observed in Group A, increased the risks for cardiac diseases. In this regard, several studies have noted worsening of the condition and even the development of cardiovascular diseases with the use of AAS [1, 11, 13].On the other hand the alterations induced by the AAS in TAG, total cholesterol and homocysteine levels, also risk factors for cardiovascular diseases [5, 26], were at normal levels in Group A. Another study observes reduction in HDL but not changes in LDL [18], demonstrating the complexity these systems interaction. However, our results corroborated those of Zmuda et al. [26], who also suggested that the AAS did not interfere in homocysteine. One of the main side effects related to the use of AAS is hepatotoxicity, including elevated blood levels of these enzymes [11]. In Group A, the levels of hepatic enzymes AST and ALT were elevated, begin significantly different compared with Group C as regards AST, and to the other two groups in the case of ALT, denoting a degree of hepatotoxicity.The creatinine increases in Group A was expected [11], but was discreetly and with significance only in comparison with Group C. On the other hand, urea was shown at higher levels in Group A compared with the other groups. Because it is the end product of protein metabolisms, this alteration is related to the increased organic needs with the athlete’s routine and the use of AAS increases the demand for muscle protein synthesis and in protein intake [17]. The physical activity is a protective factor against renal disease [32]. But, the side effects of AAS on this system have also been documented [11], and although rare, they are only partially explained by the elevation of the variables discussed in the previous paragraph. According to Daher et al. [33], acute kidney failure is not frequently reported in AAS users, although in the majority of cases this would be derived from hypercalcemia, resulting from volume depletion and renal vasoconstriction. The results are limited and confounding, since they are obtained from the combination of different drugs protocols [11].Therefore, the main limitations of the present study were due to the small n, dynamic action of the use of ergogenic resources, should be cautious in generalizing. However, these are difficult conditions to achieve due to the use of different types and doses of AAS/GH [5], particularities of the training sessions, the athletes' adhesion to the study [3] and confidence in the answers, one time that there are banned substances and that have social reprobation [2]. In this context, although we explained all the criteria concerning secrecy/confidentiality of the information, over a year was needed to convince only eight bodybuilders to participate and schedule collections of the required blood samples. Another seven athletes scheduled to participate did not appear for the collections and other exams programmed, such as the electrocardiogram, map, echocardiogram and carotid Doppler were not performed, because we were unable to get the bodybuilders to appear on another day for collection. The strong point of the study was the use of a group who did similar training to that of the bodybuilding, but without the use of AAS/GH, and a sedentary group without exercise and without drugs, thereby neutralizing the effect of exercise per se, as a biochemical modulator.
5. Conclusions
- The chronic and prolonged use of AAS/GH is a risk factor for cardiovascular, renal and hepatic disease, suggested by the biochemical alterations observed in the present study. However, these alterations were of a smaller magnitude when compared with some other studies [12, 13, 34] and partially with others that did not present alterations of significance to health [8, 14, 15, 17]. Therefore, there are still gaps in the knowledge about the effects of AAS/GH used by athletes [3], and which shall be pursued in future better controlled studies with functional measurements, and experimental counter proof. It is imperative to adopt policies that may disclose and prevent the effects of the indiscriminate use of AAS/GH in particular by the public that attends health clubs.
ACKNOWLEDGEMENTS
- Financing: Personal Improvement Coordination for Higher Education - CAPES, and; National Scientific and Technological Development Council – CNPq.
References
| [1] | Thevis M, Kuuranne T, Geyer H, et al. Annual banned-substance review: analytical approaches in human sports drug testing. Drug Test Anal 2017; 9: 6–29. |
| [2] | Angoorani H, Halabchi F. The misuse of anabolic-androgenic steroids among Iranian re-creational male body-builders and their related psycho-socio-demographic factors. Iran J Public Health 2015; 44: 1662–1669. |
| [3] | Barbalho M de SM, Barreiros FP. The Use of Anabolic- Androgenic Steroids in Sports. Sport Med 2015; 5: 171–179. |
| [4] | Mhillaj E, Morgese MG, Tucci P, et al. Effects of anabolic-androgens on brain reward function. Front Neurosci 2015; 9: 1–13. |
| [5] | Kanayama G, Pope HG. History and epidemiology of anabolic androgens in athletes and non-athletes. Mol Cell Endocrinol; in press. Epub ahead of print 2017. DOI: 10.1016/j.mce.2017.02.039. |
| [6] | Kim T, Kim YH. Korean national athletes’ knowledge, practices, and attitudes of doping: a cross-sectional study. Subst Abuse Treat Prev Policy 2017; 12: 7. |
| [7] | Boone J, Lambert C, Flynn M, et al. Resistance Exercise Effects on Plasma Cortisol, Testosterone and Creatine Kinase Activity in Anabolic-Androgenic Steroid Users. Int J Sports Med 1990; 11: 293–297. |
| [8] | Georgieva KN, Boyadjiev NP. Effects of nandrolone decanoate on V̇O2max, running economy, and endurance in rats. Med Sci Sports Exerc 2004; 36: 1336–1341. |
| [9] | Saugy M, Robinson N, Saudan C, et al. Human growth hormone doping in sport. Br J Sports Med 2006; 40 Suppl 1: i35–i39. |
| [10] | Baume N, Schumacher YO, Sottas PE, et al. Effect of multiple oral doses of androgenic anabolic steroids on endurance performance and serum indices of physical stress in healthy male subjects. Eur J Appl Physiol 2006; 98: 329–340. |
| [11] | Nieschlag E, Vorona E. Doping with anabolic androgenic steroids (AAS): Adverse effects on non-reproductive organs and functions. Rev Endocr Metab Disord 2015; 16: 199–211. |
| [12] | Kafrouni MI, Anders RA, Verma S. Hepatotoxicity Associated With Dietary Supplements Containing Anabolic Steroids. Clin Gastroenterol Hepatol 2007; 5: 809–812. |
| [13] | Cocca S, Viviano M. Stevens-Johnson syndrome and abuse of anabolic steroids. J Korean Assoc Oral Maxillofac Surg 2017; 43: 57–60. |
| [14] | Fontana K, Oliveira HCF, Leonardo MB, et al. Adverse effect of the anabolic-androgenic steroid mesterolone on cardiac remodelling and lipoprotein profile is attenuated by aerobicz exercise training. Int J Exp Pathol 2008; 89: 358–366. |
| [15] | Kara M, Ozcagli E, Fragkiadaki P, et al. Determination of DNA damage and telomerase activity in stanozolol-treated rats. Exp Ther Med 2017; 13: 614–618. |
| [16] | Grace FM, Davies B. Raised concentrations of C reactive protein in anabolic steroid using bodybuilders. Br J Sport Med 2004; 38: 97–98. |
| [17] | Hartgens F, Kuipers H. Effects of androgenic-anabolic steroids in athletes. Sports Med 2004; 34: 513–54. |
| [18] | Sader MA, Griffiths KA, McCredie RJ, et al. Androgenic anabolic steroids and arterial structure and function in male bodybuilders. J Am Coll Cardiol 2001; 37: 224–230. |
| [19] | Veras K, Silva-junior FL, Lima-silva AE, et al. Case report impaired physical performance and clinical responses after a recreational bodybuilder’s self-administration of steroids: A case report. World J Mens Health 2015; 33: 209–213. |
| [20] | Alizade E, Avci A, Tabakcı MM, et al. Comparison of Right Ventricle Systolic Function between Long-Term Anabolic–Androgenic Steroid User and Nonuser Bodybuilder Athletes: A Study of Two-Dimensional Speckle Tracking Echocardiography. Echocardiography 2016; 33: 1178–1185. |
| [21] | Powrie JK, Bassett EE, Rosen T, et al. Detection of growth hormone abuse in sport. Growth Horm IGF Res 2007; 17: 220–226. |
| [22] | Lemieux I, Lamarche B, Couillard C, et al. Total Cholesterol/HDL Cholesterol Ratio vs LDL Cholesterol/HDL Cholesterol Ratio as Indices of Ischemic Heart Disease Risk in Men. Arch Intern Med 2001; 161: 2685. |
| [23] | Gallart T, Escudero MF, Torres-puchol FG, et al. Tablas de referencia y valores normales de las pruebas de laboratorio habituales. Apéndice 2013; 12–22. |
| [24] | Souza L da CM, da Cruz L, Cerqueira E de MM, et al. Micronucleus as biomarkers of cancer risk in anabolic androgenic steroids users. Hum Exp Toxicol 2017; 36: 302–310. |
| [25] | Hislop MS, Ratanjee BD, Soule SG, et al. Effects of anabolic-androgenic steroid use or gonadal testosterone suppression on serum leptin concentration in men. Eur J Endocrinol 1999; 141: 40–46. |
| [26] | Zmuda JM, Bausserman LL, Maceroni D, et al. The effect of supraphysiologic doses of testosterone on fasting total homocysteine levels in normal men. Atherosclerosis 1997; 130: 199–202. |
| [27] | Tsai EC, Matsumoto AM, Fujimoto WY, et al. Association of Bioavailable, Free, and Total Testosterone with Insulin Resistance: Influence of sex hormone-binding globulin and body fat. Diabetes Care 2004; 27: 861–868. |
| [28] | Fett CA, Petricio A, Correa C, et al. Suplementação de Ácidos Graxos Ômega-3 ou Triglicerídios de Cadeia Média para Indivíduos em Treinamento de Força. 2001; 7: 83–91. |
| [29] | Peake JM, Tan SJ, Markworth JF, et al. Metabolic and hormonal responses to isoenergetic high-intensity interval exercise and continuous moderate-intensity exercise. AJP Endocrinol Metab 2014; 307: E539–E552. |
| [30] | Fett CA, Fett WCR, Maestá N, et al. A suplementação de ácidos graxos ômega 3 e triglicérides de cadeia média não alteram os indicadores metabólicos em um teste de exaustão. Rev Bras Med do Esporte 2004; 10: 44–49. |
| [31] | Schwingel PA, Cotrim HP, Salles BR, et al. Anabolic-androgenic steroids: A possible new risk factor of toxicant-associated fatty liver disease. Liver Int 2011; 31: 348–353. |
| [32] | Stump CS. Physical Activity in the Prevention of Chronic Kidney Disease. Cardiorenal Med 2011; 1: 164–173. |
| [33] | Daher EF, Silva Júnior GB, Queiroz AL, et al. Acute kidney injury due to anabolic steroid and vitamin supplement abuse: Report of two cases and a literature review. Int Urol Nephrol 2009; 41: 717–723. |
| [34] | Kasikcioglu E, Oflaz H, Arslan A, et al. Aortic elastic properties in athletes using anabolic-androgenic steroids. Int J Cardiol 2007; 114: 132–4. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML