-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Sports Science
p-ISSN: 2169-8759 e-ISSN: 2169-8791
2017; 7(2): 94-98
doi:10.5923/j.sports.20170702.11

Association of Serum Lactate Dehydrogenase, Creatinine Kinase and Adiposity with Basal Lactate Concentration in Male and Female Jamaican Athletes
Aldeam Facey1, Lowell Dilworth2, Rosemarie Wright-Pascoe3, Rachael Irving1
1The Department of Basic Medical Sciences, University of the West Indies, Mona Campus
2The Pathology Department of Pathology, University of the West Indies, Mona Campus
3The Department of Medicine, University of the West Indies, Mona Campus
Correspondence to: Aldeam Facey, The Department of Basic Medical Sciences, University of the West Indies, Mona Campus.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

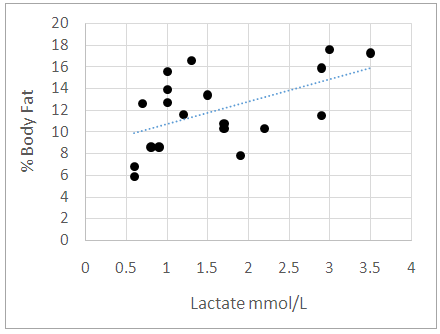
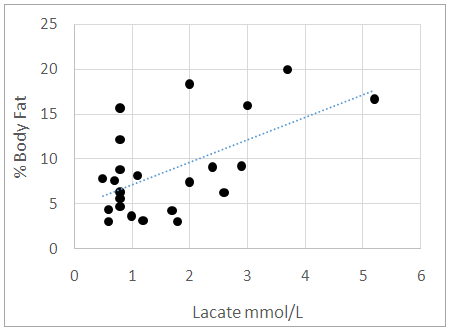
Research has shown that basal lactate increases with body fat in males of West Indian descent. The relationship in females of West Indian descent has however not been clearly elucidated. Lactate Dehydrogenase (LDH) and creatinine (CK) are associated with energy metabolism and are involved in fat storage. Since basal lactate increases with adiposity, these enzymes may impact the regulation of basal lactate more significantly than fat. The study seeks to establish the relationship between adiposity and fasting or resting serum lactate (basal lactate) in male and female athletes and to ascertain if the concentrations of these enzymes contribute to the level of basal lactate. In this study of 23 male and 10 female athletes and 19 males and 17 female non-athletes, fasting or resting basal lactate, body fat percentage and concentration of CK and LDH were evaluated. Basal lactate was determined using a handheld lactate analyser and body fat percentage was determined using ultrasound technology. The concentration of the enzymes CK and LDH were also measured from serum. Results revealed that lactate increases with fat level in males and female’s athletes and non-athletes. Fat > 29% did not contribute to increased serum basal lactate concentration in females. Basal lactate concentration was not significantly impacted by the concentrations of CK and LDH. Body fat can be used to estimate basal lactate concentration in blood in male and female athletes. A post exercise assessment of serum CK and LDH and impact on basal lactate is warranted.
Keywords: Creatinine Kinase, Lactate Dehydrogenase, Basal Lactate
Cite this paper: Aldeam Facey, Lowell Dilworth, Rosemarie Wright-Pascoe, Rachael Irving, Association of Serum Lactate Dehydrogenase, Creatinine Kinase and Adiposity with Basal Lactate Concentration in Male and Female Jamaican Athletes, International Journal of Sports Science, Vol. 7 No. 2, 2017, pp. 94-98. doi: 10.5923/j.sports.20170702.11.
1. Introduction
- Glycolysis is the metabolic pathway which breaks down glucose to pyruvate producing energy in the forms of adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide (NADH) [1]. The ATP is the fuel source for muscular contraction [1]. This fuel source lasts only for a short duration during intense physical exercise and is replenished through the phosphagen system, glycolytic pathway or through mitochondrial respiration [1]. There can be a simultaneous and coordinated effort of all the energy systems to satisfy the energy demands at varying exercise intensities [1]. Lactate is a product of the glycolytic pathway, derived from pyruvate through the action of lactate dehydrogenase. A reduction in oxygen availability stimulates increased production of lactate. During muscular contraction, the levels can increase exponentially [2]. Lactate accumulation in muscles depends on a variety of physiological factors and its production is associated with muscular fatigue [2]. Lactate metabolism is very important when considering athletic performance, as the rate of accumulation can be an indicator of fitness. It has also been discovered that lactate is produced by adipocytes [3]. The level of resting serum lactate is a potential a predictor of the level of muscular fatigue during training or competition [4]. Creatinine kinase (CK) is a key enzyme in energy metabolism. There are several isoenzymes of CK which are compartmentalized where energy is used or where energy is produced [5]. There are four subunits of CK which combine to form the various isoforms [5]. The two cytosolic forms called M-CK and B-CK representing muscle and brain respectively where they are seen predominantly [5]. There are also two mitochondrial subunits called Mi-CK [5]. The two cytosolic subunits combine to form 3 isoenzymes, MM-CK, MB-CK and BB-CK [5]. CK catalyses the reversible phosphorylation of creatine in the presence of ATP and hydrogen ions [5]. The resulting organic compound is creatinine phosphate (CrP) which, at the beginning of muscular energy expenditure, phosphorylates ADP resulting in mild alkalinisation of the muscle [6].The enzyme concentration increases during myocardial necrosis and as such can be used in diagnosing this condition [7]. CK levels are higher in physically active individuals [8]. CK can also indicate tissue damage and myocardial infarction [9]. In in black people the activity of CK was found to be higher when compared to white people averaging 88 IU/L and 149 IU/L respectively [9]. In healthy people CK is believed to leak from tissues into lymphatic vessels and into the blood stream [10]. The rate of the leak is believed to be proportional to intracellular CK concentration [10]. The literature does not define a clear relationship between serum CK and serum basal lactateThe conversions of pyruvate to lactate and vice versa are governed by the enzyme lactate dehydrogenase [11]. Two subunits combine to form the enzyme; the muscle (M) subunit and the heart (H) subunit [11]. Lactate dehydrogenase 5 (LDH-5) consists of only muscle subunits while lactate dehydrogenase 1 (LDH-1) consists of only heart subunits [11]. The three other isoforms have varying proportions of H and M subunits. The muscle -type facilitates the conversion of pyruvate to lactate and is present in glycolytic tissues [11]. The heart- type favours the production of pyruvate from lactate and has a lower maximal velocity [11]. Elevated serum LDH levels, like CK, can indicate muscle damage [12-14].Given the y involvement of CK and LDH in energy metabolism. It is our hypothesis that the activities/concentrations of serum CK and LDH are associated with basal lactate concentration. The study was designed to evaluate fasting basal lactate, body fat percentage and the concentrations of serum CK and LDH in athletes and non-athletes. Research has shown that serum basal lactate in male athletes is associated with fat and as such can be used as a fitness assessment tool [4]. The study aimed to look at percentage fat level and other factors that may contribute to serum basal lactate concentration. This is necessary to determine basal lactate validity and reliability as a fitness assessment tool in males and to try to replicate the assessment in the females n.
2. Method
- Ethical approval was granted by the University Hospital/ University of the West Indies Ethics Committee for conduct of the research. Athletes were selected from the University of the West Indies Mona track and field team as well as a track club whose members train on the University of the West Indies Campus. The non-athletes were volunteer students from the University of the West Indies, Mona. Signed, informed consent was obtained from each participant. Four groups were selected for this study. The groups included 23 male athletes and 19 controls and 10 female athletes and 17 controls. Ages of participants varied between 19 and 32 years old. Alcoholics and smokers were excluded from this study. Athletes were confirmed to be athletes based on a physical fitness questionnaire which was administered to all subjects recruited. A modified American Council on Exercise physical fitness questionnaire [7] was used as a tool to distinguish the athletes from the controls. The questionnaire was modified to include past and present medical history. Each athlete trained 5 days each week with a certified coach while the controls did little to no exercise with less than 20 minutes of physical activity per week for at least 6 months prior to the assessment. The participants were asked to fast and rest overnight before the start of the assessment and samples collected the following mornings. The resting pulse rate and blood pressure while seated were recorded three consecutive times using an electronic sphygmomanometer [16]. Anthropometric measurements inclusive of waist circumference, hip circumference, height and weight were taken. Body fat percentages were measured using the BodyMetrix Analyzer. This method utilized the three-site technique which employed a modified version of the Jackson-Pollock three site skinfold equation for males and the Pollock three site equation for the females [17]. The Machine measures the thickness of the fat at the specific site by use of ultrasound technology [18]. Three points on the right side of the body were used. For males, the ultrasound machine was placed at the chest mid-way between the nipple and the shoulder joint. A read out was displayed on a computer which recorded the thickness of each tissue that is fat, muscle and the interfaces in millimetres. This was done 2 to 3 times and an average for improved accuracy was determined. This was repeated at the two other areas, one an inch right of the umbilicus and the other on the thigh mid-way between the knee and hip joints. For females, the sites used are: mid right triceps, the right hip an inch above the pelvic bone and an inch right of the umbilicus. From the readings obtained the percentage body fat was calculated.A Lactate Plus analyser was used to measure the serum resting lactate levels. The sample was obtained by finger-prick of the index finger. Venepuncture was used to collect a sample of blood which was tested for the activities of the enzymes CK and LDH. The Cobas 6000 autoanalyzer was used for analysis of the serum samples using spectrophotometry with reagents supplied by Roche Diagnostics (Roche Diagnostics, Indianapolis, USA). Data AnalysisData were analysed using the Pearson's bivariate correlation, a two-tailed t-test and the level of significance was noted at the P≤0.05 level. Data is generally reported as mean ±SD.
3. Results
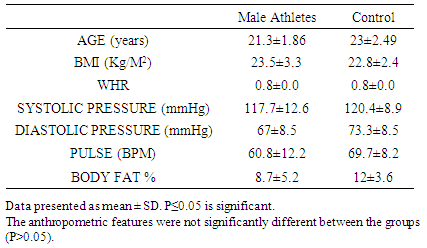
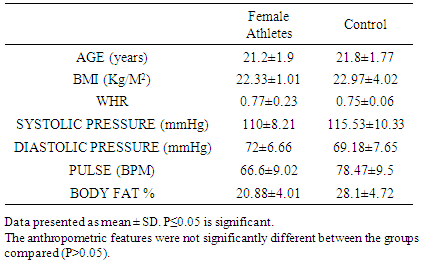
- The results showed no significant anthropometric differences between the athletes and control groups in their ages, body mass index, waist –hip-ratio blood pressures (systolic and diastolic) and in their pulse rates (P>0.05). The only he primary difference between the groups was the level of physical activity (Tables 1 and 2).
|
|
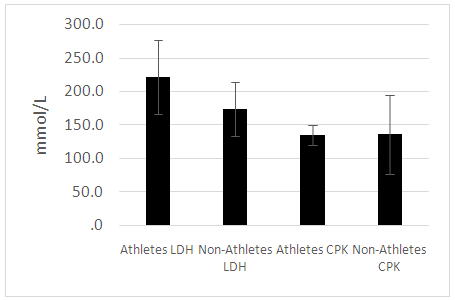
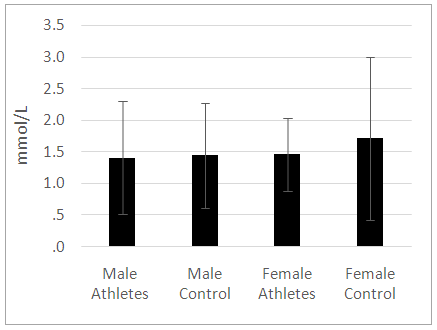 | Figure 1. A comparison of the basal lactate concentration between the athletes and controls. No significant difference was noted in serum lactate concentration among the groups assessed (P>0.05) |
4. Discussion
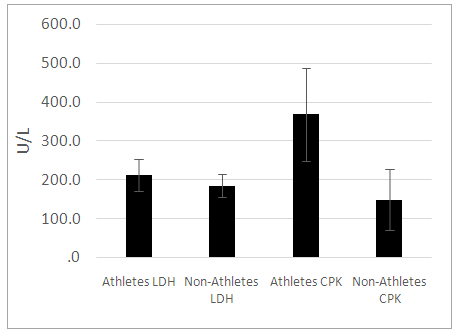
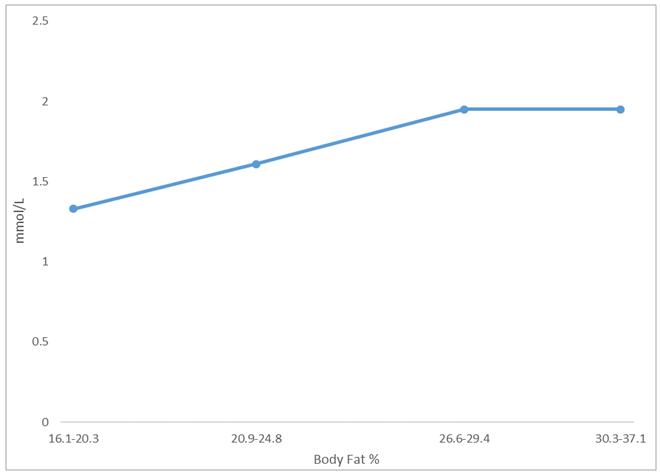
- Minimal anthropometric difference between the athletes and controls were noticeable. As there was no major anthropometric difference between the athletic and control groups, we can credibly compare the effects of the enzymes measured. Previous study documented that lactate increases with fat in the males of West African descent [4]. Our results were similar. Furthermore, our study documented that this relationship is also true for the female athletes but not controls. We noted in our study that the female controls assessed had on average higher body fat percentage while the female athletes’ body fat percentages ranged from low to high. This may explain the lack of significant correlation between adiposity and basal lactate that was observed in the female control population. For a relationship between basal lactate and fat to be established, there needs to be more variation in body fat within the group. If the group consists of only individuals with high body fat, who all have high serum basal lactate concentrations, it will be difficult to make any correlation. The ranges of fat were assessed in the total female population and it was found that lactate increased with fat until body fat exceeded 29%. We concluded that in females, there is a positive proportional relationship between fat and serum basal lactate until increased fat no longer contributes to the serum basal lactate concentration. Ideally, another study should be done to explore further this relationship by using obese men to determine if the same is true for the male population. CK was elevated in the male athletes but not in the female athletes. This is suggestive possibly of muscle injuries in the male athletes or they may be high responders to physical training [8]. Serum CK and LDH did not significantly correlate with serum basal lactate concentration in neither males or females. This result contrasts with what was hypothesized as these enzymes are associated with energy metabolism [8, 19]. Many physiological factors may have accounted for the lack of correlation observed [2]. It is likely that their association will be more evident in a post exercise assessment. We suggest that the relationship between body fat and serum lactate at the basal level is independent of the activities of serum CK and LDH. This indicates greater reliability on body fat in estimating the basal lactate concentration in serum.To provide a more accurate assessment of the relationship between basal lactate in the female controls, a population with greater variation in body fat percentage is required. This information came out of the study CK catalyzes the reversible phosphorylation of creatine in the presence of ATP and hydrogen ions [5] while LDH catalyzes the conversion of pyruvate to lactate [11]. It is expected that these enzymes contribute in some way to the metabolism of lactate. To better assess the impact these enzymes can have on serum lactate concentration, a study designed to assess the relationship between changes in serum lactate concentration and changes in the activity of these enzymes during physical exercise is needed. Another suggestion is the use of an intracellular LDH assessment in lieu of serum LDH. It is possible that the only time the activities of the enzymes will correlate with the lactate concentration is during physical activity as our results indicate otherwise in the resting state.
5. Conclusions
- Serum basal lactate increased with body fat percentage in males and females. Fat, however, did not contribute to further increase in basal lactate concentration in females when the fat percentage exceeded 29%. Serum CK and LDH were not significantly associated with basal lactate concentration. Basal lactate concentration has been found to primarily depend on body fat percentage. The adipocytic contribution to basal blood lactate is a reliable assessment tool in athletes and control as it is independent of serum CK and LDH. A post exercise assessment of each serum parameter may yield valuable insight.
ACKNOWLEDGEMENTS
- The authors wish to acknowledge the Office of Graduate Studies and Research at the University of the West Indies, Mona, for funding the research.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML