-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Sports Science
p-ISSN: 2169-8759 e-ISSN: 2169-8791
2016; 6(6): 237-242
doi:10.5923/j.sports.20160606.06

Critical Load Evaluation in Male Adult Wistar Rats with Zymosan-Induced Arthritis
Juan Parente Santos, André Arnosti, Maria Izabel Camargo-Mathias
Universidade Estadual Paulista, Júlio de Mesquita Filho, UNESP, Rio Claro, S.P., Brazil
Correspondence to: Maria Izabel Camargo-Mathias, Universidade Estadual Paulista, Júlio de Mesquita Filho, UNESP, Rio Claro, S.P., Brazil.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This study aimed to verify the effects of experimentally induced arthritis on the critical load of rats submitted to swimming exercise. For this, ten male Wistar rats were divided into two groups: G1, formed by healthy rats, and G2, constituted by rats with Zymosan-induced arthritis. Zymosan is known for inducing inflammation when injected in the knee joint of rats, mice and rabbits, causing subacute proliferative arthritis characterized by persistent synovitis and the disorganization of the cartilage by inducing macrophage expression, causing prolonged inflammatory responses. Both groups were submitted to swimming exercise for two consecutive days, with critical loads obtained through physical test in water with overload proportional to the animals’ body mass. The posterior joint region of the animals was radiographically analyzed and the statistical analysis of the critical loads values was performed. The radiography showed the occurrence of slight joint alterations in the animals from group G2, possibly due to the onset of an inflammatory process, and the critical load mean value obtained for the group G2 was significantly lower (27.385 ± 0.0065) than that of G1 (35.735 ± 0.007), with p< 0.001. The data obtained in this study demonstrated that the inflammation induced in the posterior joint region of the rats from group G2 affected the performance of swimming exercise, and, consequently, the critical load value. In addition, the results showed that the joint impairment required a greater physical effort from the animals tested to perform the exercise.
Keywords: Physical exercise, Swimming, Effort intensity, Anaerobic threshold, Knee joint, Inflammation, Radiographic examination
Cite this paper: Juan Parente Santos, André Arnosti, Maria Izabel Camargo-Mathias, Critical Load Evaluation in Male Adult Wistar Rats with Zymosan-Induced Arthritis, International Journal of Sports Science, Vol. 6 No. 6, 2016, pp. 237-242. doi: 10.5923/j.sports.20160606.06.
Article Outline
1. Introduction
- Studies on exercise physiology have used animal models, especially regarding the practice of invasive manipulations. The use of mammals in laboratory research simulates physical stress conditions observed in human beings, aiming to find the most suitable follow-up procedures to observe systemic exercise alterations. [1-3] Swimming is one of the most commonly used evaluation methods, once it is an activity associated with the ergonomic cycle. Rats are the most used biological models due to their reduced size, easy handling and good response to exercise. In addition, the physiological response of these animals is similar to that of human beings [4, 5]. Moreover, the choice for this experimental model is justified by the possibility to measure injuries (in this case, the joint cartilage) and analyze the systemic biological effects that involve different therapeutic methods [6, 7].According to the literature, the effort intensity in rats can be determined through the quantification of the blood lactate or by obtaining the critical load [4].The critical load (CL), or critical power (CP), corresponds to the highest intensity at which the exercise can be performed without exhaustion; therefore, it is related with aerobic capacity. Thus, the critical load model has the advantage of determining, by mathematical method, both the aerobic capacity (CL) and anaerobic power stock (ASC = anaerobic swimming capacity) of the test organism [8, 9].The critical load parameter is defined as the highest exercise intensity that can be maintained for a considerable period, where conditions of relative metabolic balance are expressed in stable values of oxygen consumption and lactatemia. Thus, it is regarded as a parameter indicating aerobic performance capacity; i.e., the boundary between the domains of heavy and severe intensity [10-12]. The noninvasive protocol used to evaluate the critical power in Wistar rats was established by [13]. The animals were submitted to four tests with loads (L) ranging from 9% to 15% of their body mass on consecutive days. The time to exhaustion (tlim) was determined for each intensity and applied to the hyperbolic equation, linearly adjusted by the function: Load= CL + ASC.1/tlim.According to literature, the experimentally induced arthritis is similar to the naturally occurring in human beings. Several substances can be used to induce arthritis, such as adjuvants, streptococcal cell wall fragments, type II collagen, retroviruses, lactobacilli, mycoplasma, mycobacteria [14-16] and Zymosan (Zy) [17-19]. Zymosan is a polysaccharide derived from the cell wall of Saccharomyces cerevisae (baker’s yeast), and is known for inducing inflammation when injected in the knee joint of rats, mice and rabbits, causing subacute proliferative arthritis characterized by persistent synovitis and cartilage degradation as well, reproducing most of the symptoms observed in human beings. In addition, studies using Zymosan showed that periarticular tissues and regions suffer alterations similar to those caused by autoimmune diseases [20, 21].Considering this information, the present study had the objective to compare the critical load values imposed to healthy male adult Wistar rats and those with Zymosan-induced arthritis to verify the influence of the disease on the swimming exercise critical load.
2. Material
- In this experiment, ten male adult Wistar rats (150 days oldweighing approximately 500g were used. The rats were kept in collective polypropylene cages in the facilities of the Biodynamics Laboratory of the Physical Education Department from UNESP campus Rio Claro (SP) at controlled temperature (22°C±2°C), 12h photoperiod, receiving water and food ad libitum. The animals were divided into two groups: a) G1: five healthy individuals, and b) G2: five individuals with induced arthritis. Both groups were submitted to swimming exercise, critical load evaluation and radiographic examination to detect the level of inflammation.This experiment was approved by the Ethical Committee on Animal Use (ECAU), UNESP/Rio Claro, SP, protocol number 005957.
3. Methods
3.1. Inoculation
- The inoculation procedure was performed in the Biodynamics Laboratory of the Physical Education Department, UNESP, campus Rio Claro, SP. Rats from group G2 were anesthetized in time 0 (T0) with ketamine hydrochloride (80mg/kg MC/IP) xylazine hydrochloride (20mg/kg MC/IP) and inoculated once with an intra-articular injection with 0.1 mL/rat of Zymosan - Sigma (Saccharomyces cerevisae) solution containing 1mg of Zymosan/0.5 mL of saline solution 0.9%.The disease onset occurred on the 3rd day and the physical activity intervention started on the 15th day after inoculation.The anesthetizing and inoculation procedures were supervised by the veterinary physician Letícia Maria Graballos Ferraz Hebling, CRMV – SP 5.412, collaborator to the research group led by Prof. Dr. Maria Izabel Camargo Mathias, responsible for the development of this study.
3.2. Radiographic Examination
- The radiographic examination was performed in the veterinary clinic Polivet located in Rio Claro (SP), under the supervision of the licensed veterinary Letícia Maria Graballos Ferraz Hebling CRMV/SP 5.412. Simple, latero-lateral and anteroposterior radiographic images were produced at the intensity of 40KV/100mA.The animals from groups G1 and G2 were radiographed in time T9 (9 days after the inoculation) to verify the presence and level of inflammation caused by the arthritis induced on the rats belonging to group G2.
3.3. Determination of Critical Load (CL) [22]
- The whole training was performed in the Biodynamics Laboratory of the Physical Education Department, UNESP campus Rio Claro in times T11 and T12 (11th and 12th day after inoculation). A polypropylene box 60cm x 75cm x 115cm) was filled with approximately 350 L of water at 31°C±2°C. A PVC tube measuring 60 cm x 10 cm was attached to the box, where the rats were placed one by one to swim. With this methodology, each animal was analyzed individually, allowing an accurate observation to obtain the times to exhaustion.After each swimming bout, the animals were dried with a cotton microfiber towel and returned to the cages.
3.4. Adaptation to Water
- Prior to the experiment, the animals were adapted to water for five consecutive days, The purpose of the adaptation was to reduce stress without promoting exercise training adaptations. The following procedure was adopted: a) on the first day the animals were placed in shallow water for 5 minutes; b) on the second day, the rats remained in the water for 10 minutes; c) on the third day the animals were placed in deep water, individualized in tubes, remaining for 5 minutes; d) on the fourth day the rats remained in deep water, in tubes, for 10 minutes; e) on the last day the animals were placed in deep water, in tubes, for 5 minutes with an overload corresponding to 3% of their body weight (BW).
3.5. Overload Training
- After the adaptation, the animals were submitted to two swimming tests with loads tied to their back (lead fish sinkers), corresponding to 9%, 11%, 13% and 15% of their body weight (BW).This test consisted in submitting the rats to exhaustion for intercalated periods with different loads. In the morning of the first day, a 9% load was used for each rat. In the afternoon of the same day, a 13% load was used. In the morning of the second day, a 11% load was used, and, in the afternoon, a 15% load. The times to exhaustion (tlim) for each rat with the respective loads were recorded and inserted in the hyperbolic equation to swimming rats, where x = 1/Tlim (Tlim= time limit to exhaustion) and y = load (% BW), which provided the critical load value per rat (CL/rat) [13] adapted by [23].Finally, the critical load means for the groups were obtained and statistically analyzed using ANOVA test with TUKEY post-test. Differences with p<0.05 were not considered significant.
4. Results
4.1. Radiographic Evaluation
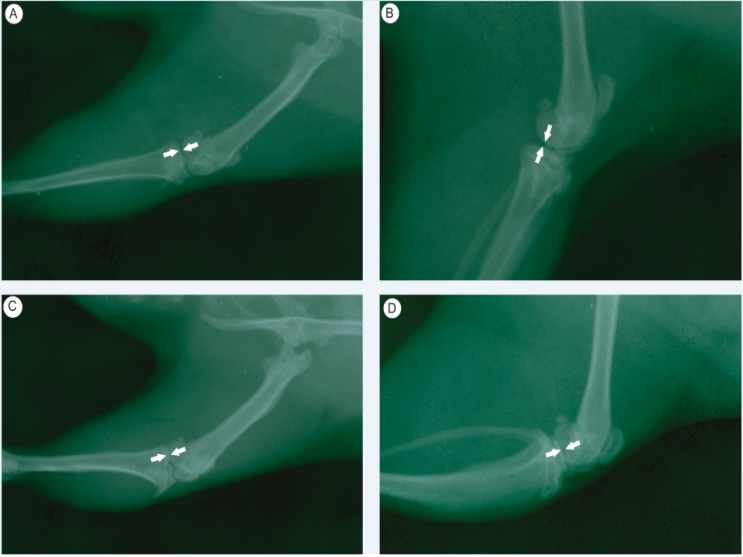
- The results obtained through the radiographic images showed that the articulations of the rats from group G1 were intact and did not present radiopacity. (Fig. 1A e B). Contrarily, the individuals from group G2, presented slight inflammation and a discreet increase in the periarticular radiopacity of the joint capsule, mainly in the craniodistal portion (Fig. 1C and D).
4.2. Critical load (CL) Evaluation
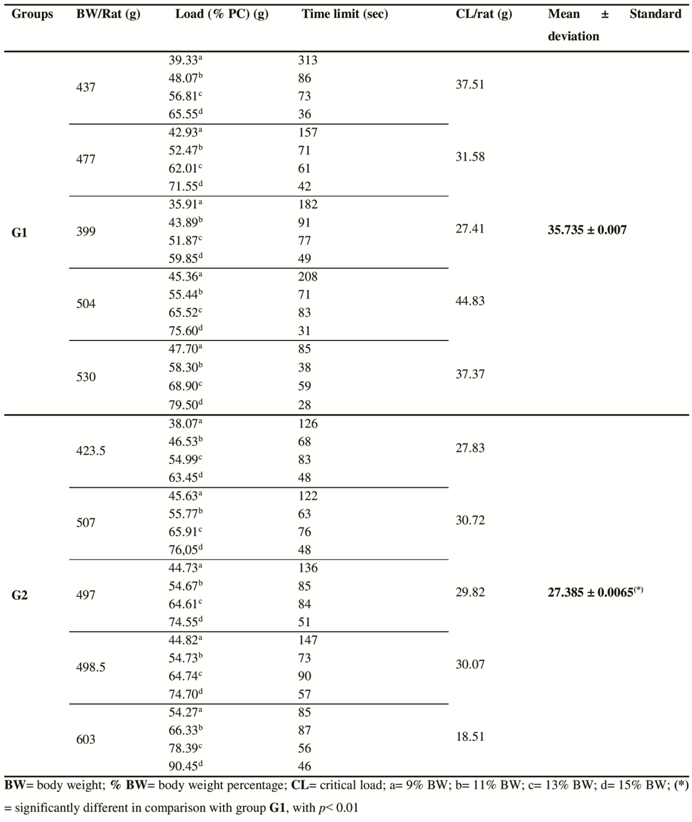
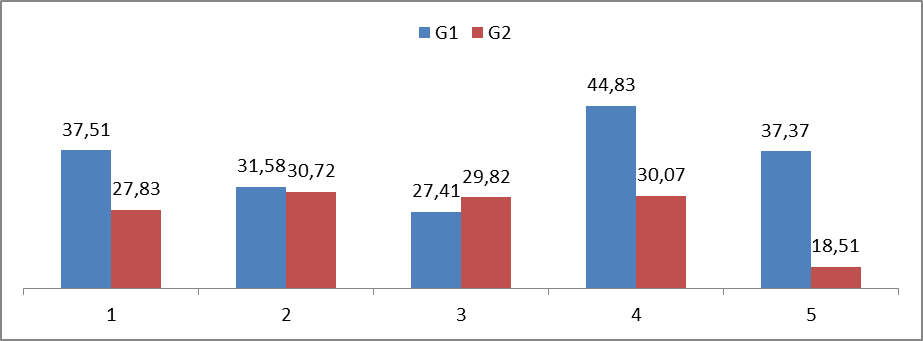
- The evaluation of the critical load obtained from 5 individuals from group G1 and 5 individuals from group G2 showed that the respective means and standard deviations were significantly lower in the individuals from group G2 (27.385±0.0065) in comparison with the ones from group G1 (35.735±0.007) with p<0.01. For better visualization, the data is plotted on Table 1.
|
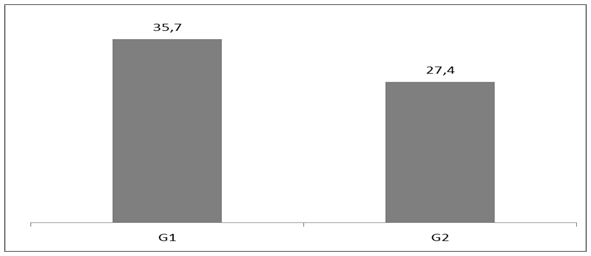 | Figure 2. Graphic representation comparing G1 and G2 critical load mean values |
5. Discussion
- This study analyzed male adult Wistar rats with Zymosan-induced arthritis aiming to investigate (through radiographic analysis) the onset of the inflammation in the knee joints of the individuals and the influence of such alteration on the critical load (CL); i.e., the ideal intensity to be applied on the exercise, in this case, swimming.The results showed that the critical load values for the rats with induced arthritis (group G2) were significantly lower in comparison with the values obtained for the healthy animals (group G1). It was clearly demonstrated that the inflammation affected the exercise performance, and, consequently the critical load. In addition, the effort required from the animals with articulation impairment was higher in comparison with the individuals belonging to the control group, indicating that these individuals should be saved in terms of motor performance. These data corroborate the literature, showing that rats with induced arthritis present evident morphological alterations, such as edemas and joint amplitude limitations, which would prevent the performance of simple motor activities; e.g., locomotion on solid ground. Symptoms as acute pain, joint swelling and stiffness were also observed, causing consequent alteration in the performance of the physical activity and difficulty in locomotion. Several studies on arthritis have demonstrated that the disease affects the joint lining (inflammation of the synovial membrane), making the joints hypertrophic and causing significant cartilage damage, once the friction between the bones is considerably increased.The radiographic data obtained here showed that the level of inflammation in the knee joint region of the rats from G2 was relatively mild, once the amount of Zymosan solution injected was four times lower than that recommended in the literature [21]. This procedure was performed to show that an articular inflammation process, even mild, is able to affect the swimming critical load parameter. Therefore, it can be suggested that the prescription of exercise intensity and volume shall be determined on an individual basis [24]. The critical load mean value obtained for group G1 (35.735 ± 0.007) was significantly higher than the one obtained for the group G2 (27,385 ± 0.0065). This demonstrates that these values would be directly proportional to the inflammation level and to the osteoarticular impairment occurred in these rats, once the healthy animals reached the time to exhaustion much later than the ones from the group with induced arthritis.Reference [25] has demonstrated that long low-intensity swimming training could bring benefits to rats with formalin-induced inflammation. Some clinical studies have suggested that physical exercise would be able to reduce the chronic pain associated with fibromyalgia, osteoporosis, inflammation of the facial/neck muscles, and cancer [26-32]. Contrarily, others reported that physical exercise would increase the chronic pain associated with fibromyalgia [33] and chronic fatigue syndrome [34], which signalizes the need of further research establishing the ideal conditions to optimize the results of the existing therapeutic methods.Thus, the data obtained here demonstrated that the symptoms articulation pathologies, mainly in the inferior limbs (rheumatoid arthritis) could be relieved with the association of pharmacological treatment and assisted physical activity, prescribed on an individual basis, considering the most suitable type of activity, volume and intensity for each individual in order to obtain relevant and satisfactory results.
ACKNOWLEDGEMENTS
- The authors would like to thank the CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) which contributed to the realization of this study through research fellowship to Dr. Maria Izabel Camargo-Mathias. Furthermore, this work has been encouraged by the Instituto Federal de Educação, Ciência e Tecnologia da Paraiba (IFPB).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML