-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Sports Science
p-ISSN: 2169-8759 e-ISSN: 2169-8791
2016; 6(5): 195-202
doi:10.5923/j.sports.20160605.05

Acute Effects of Different Strength Training Protocols on Arterial Stiffness in Healthy Subjects
Nico Nitzsche, Martin Weigert, Lutz Baumgärtel, Tino Auerbach, Daniel Schuffenhauer, Robert Nitzsche, Henry Schulz
Institute of Human Movement Science and Health, Technische Universität Chemnitz, Chemnitz, Germany
Correspondence to: Nico Nitzsche, Institute of Human Movement Science and Health, Technische Universität Chemnitz, Chemnitz, Germany.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Strength training plays an important role in cardiovascular prevention and rehabilitation. Studies showed inconsistent chronic and acute effects of strength training on arterial stiffness as an emerging biomarker of vascular health. Using pulse wave analysis, the arterial stiffness can be quantified by calculating the pulse wave velocity (PWV). The present study compared acute effects of three strength training protocols on arterial stiffness. 41 healthy, physically active subjects (age 23.8±2.3 yr, height 1.78±0.1 m, body weight 72.9±9.0 kg, body mass index 22.9±2.0 kg/m2) were assigned to three groups: group 1: 30% one repetition maximum (1RM), 3x30 repetitions, group 2: 50% 1RM 3x20 repetitions, group 3: 70% 1RM, 4x10 repetitions. All groups completed a resistance exercise session with five dynamic exercises. Pulse wave velocity (PWV), central diastolic (cDBP) and central systolic blood pressure (cSBP) were measured at rest, 0, 5 and 10 minutes after the training session with a pulse wave analysis system. PWV and cSBP showed an increase after resistance training in group 1 and 2 (p<0.05) but not in group 3 (p>0.05). cDBP decreased in all groups 5 and 10 minutes after training compared to 0 minutes after training. These results indicate that resistance exercise with low, moderate and high intensities reduce cDBP 5 and 10 minutes after a training session, but only those protocols with lower load and more repetitions acutely increase arterial stiffness in healthy subjects.
Keywords: Pulse wave velocity, Central blood pressure, Arterial stiffness, Strength training, Training protocols
Cite this paper: Nico Nitzsche, Martin Weigert, Lutz Baumgärtel, Tino Auerbach, Daniel Schuffenhauer, Robert Nitzsche, Henry Schulz, Acute Effects of Different Strength Training Protocols on Arterial Stiffness in Healthy Subjects, International Journal of Sports Science, Vol. 6 No. 5, 2016, pp. 195-202. doi: 10.5923/j.sports.20160605.05.
1. Introduction
- Strength training is highly recommended for prevention and treatment of cardiovascular diseases [1–3]. Relevant positive effects of strength training on cardiovascular risk parameters were detected [4, 5].A significant predictor of cardiovascular risk is the arterial stiffness [6, 7]. Using pulse wave analysis (PWA), the arterial stiffness can be quantified by calculating the pulse wave velocity (PWV). The decreasing elasticity from central to peripheral arteries leads to an increase of PWV, systolic blood pressure (SBP) and of pulse pressure (PP) from central to peripheral arteries [7]. However, the central systolic blood pressure (cSBP) and the central pulse pressure (cPP) are better associated with end-organ damage than the SBP and PP measured oscillometrically at the upper arm [6, 8, 9]. Central PWV has proved to be a good predictor for cardiovascular events [10]. According to the recent consensus paper on arterial stiffness of the European Society of Hypertension and Cardiology, a PWV equal or above 10 m/s is regarded as a manifested end-organ damage.Studies that have investigated the chronic influence of several weeks of physical training on arterial stiffness have demonstrated the positive effects of endurance training on the PWV and central pulse pressure [11], [12]. With regard to strength training interventions, meta-analysis have shown results with discrepancy. Ashor et al. [11] concluded that strength training has no chronic effect on PWV, but there was a significant heterogeneity between the studies. Intense strength training leads to a chronic increase of the PWV [13, 14], but Rossow et al. [15] could not identify any effect on the PWV with 80% of 1RM with an 8-week strength training. However, resistance exercise seems to have no adverse effect on arterial stiffness if the training is performed with low intensity or in a slow eccentric manner or with lower limbs [13]. In one study, a weight training with low intensity reduced PWV after 10 weeks and improved vascular elasticity [16]. Changes in PWV following chronic resistance training were not related to changes in heart rate or blood pressure after interventions [11]. There is less knowledge about acute effects of exercise on vascular elasticity. For an individual risk factor oriented control of a strength training, acute cardiovascular responses to exercise are of major relevance. This is especially important for target groups that have cardiovascular diseases or that have an increased risk of developing cardiovascular disease due to metabolic disorders (diabetes mellitus, renal failure). Therefore, it is crucial to understand the acute response of arterial stiffness to a strength training. Some studies have demonstrated an increased PWV after a strength training involving all major muscle groups [17–19]. After less than 60 minutes the PWV was back to baseline level [17]. Furthermore intensive resistance exercises of the upper body elevated PWV significantly [20]. Nevertheless, another study showed no significant changes in PWV after strength training [21]. A single-leg resistance exercise appeared to decrease arterial stiffness in the exercised leg while having no effect on central arterial stiffness or arterial stiffness of the non-exercised leg [22]. However, the exercise intensity and the measurement times were very different in all studies. Only one of the existing studies examined the PWV immediately after strength training [23] and none of the studies distinguished between intensity levels. In our own studies, an increase in arterial stiffness during isometric muscle tension was observed [24]. Recent studies have examined the physiological response of vascular stiffness during strength training. Strictly standardized experimental protocols were chosen, which examined the physiological effects. No study compared different training protocols that are applied in training practice. The purpose of the present study was to examine the acute effects of three practice-oriented strength training protocols with different intensity levels and exercise volumes on arterial stiffness and on central blood pressure parameters.
2. Methods
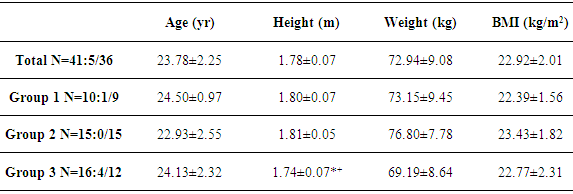
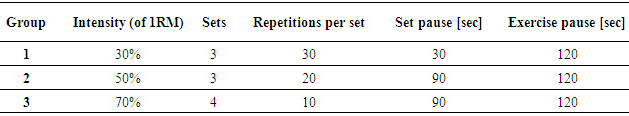
- 41 healthy sport students (5 women, 36 men; age 23,8±2,3 yr; height 1,78±0,1 m; body weight 72,9±9,0 kg; BMI 22,9±2,0 kg/m2) were assigned into three groups (Table 1) with different strength exercise protocols (Table 2). All of the participants have been physically active in one or more kind of sport for at least two years. All were normotensive, non-obese and free of known cardiovascular or metabolic disease. No participant took medications likely to affect the heart rate (HR) or the blood pressure (BP). Participants were non-smokers and were instructed to avoid alcohol, caffeine and strenuous exercise 24-h before the testing. All participants gave written informed consent. This study conformed to the principles outlined in the Helsinki Declaration.
|
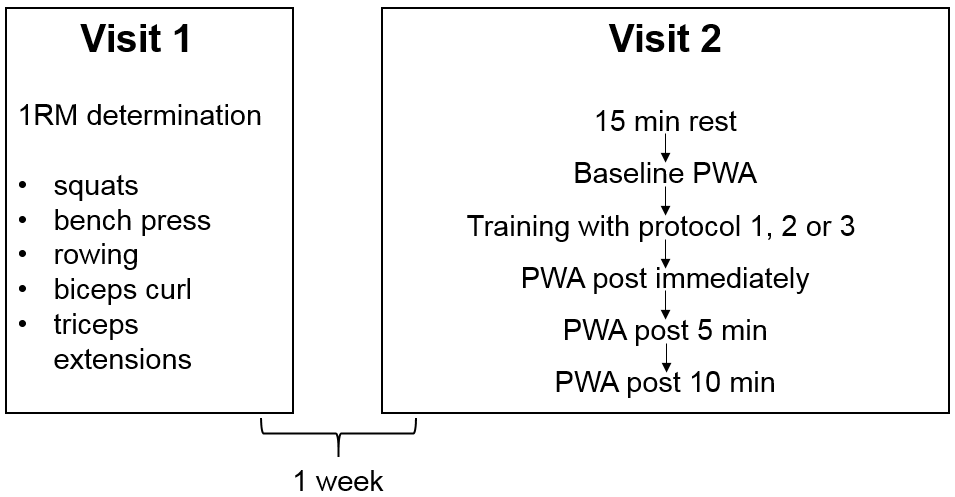 | Figure 1. Flow diagram of the study design (1RM = one repetition maximum, PWA = pulse wave analysis) |
3. Results
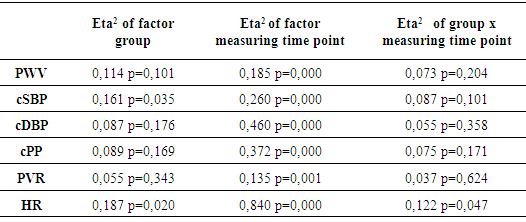
- Table 3 shows the average values as well as the standard deviations of the studied parameters. No significant differences of PWV among different exercise protocols could be detected (p > 0,05). The largest time effects within the groups showed the measurements immediately after training with a significant increase in PWV, cSBP, cPP and HR compared to the resting value. In Figure 2, it is evident that the training protocol of group 3 (high intensity, low repetition number, long set pause) leads to the lowest deflections of the measured parameters. It was the only training protocol without significant changes in the PWV and the cSBP at different time points. The rest values of these parameters were reached after 5 minutes, while in group 1 and 2 the rest values were not reached again after 10 minutes. Group 3 showed also the significantly smallest increase of the HR. The exercise protocol effected only the HR (partial Eta² = 0,122; p = 0,047) and the cSBP (partial Eta² = 0,161; p = 0,035). The other parameters showed no significant effect between the groups (p > 0,05). The factor “measuring time point” influenced all parameters (Table 4). The analysis of variance with covariate cSBP showed a significant effect on the PWV (p < 0.05). This means that load dependent increases of the blood pressure also increase the PWV.
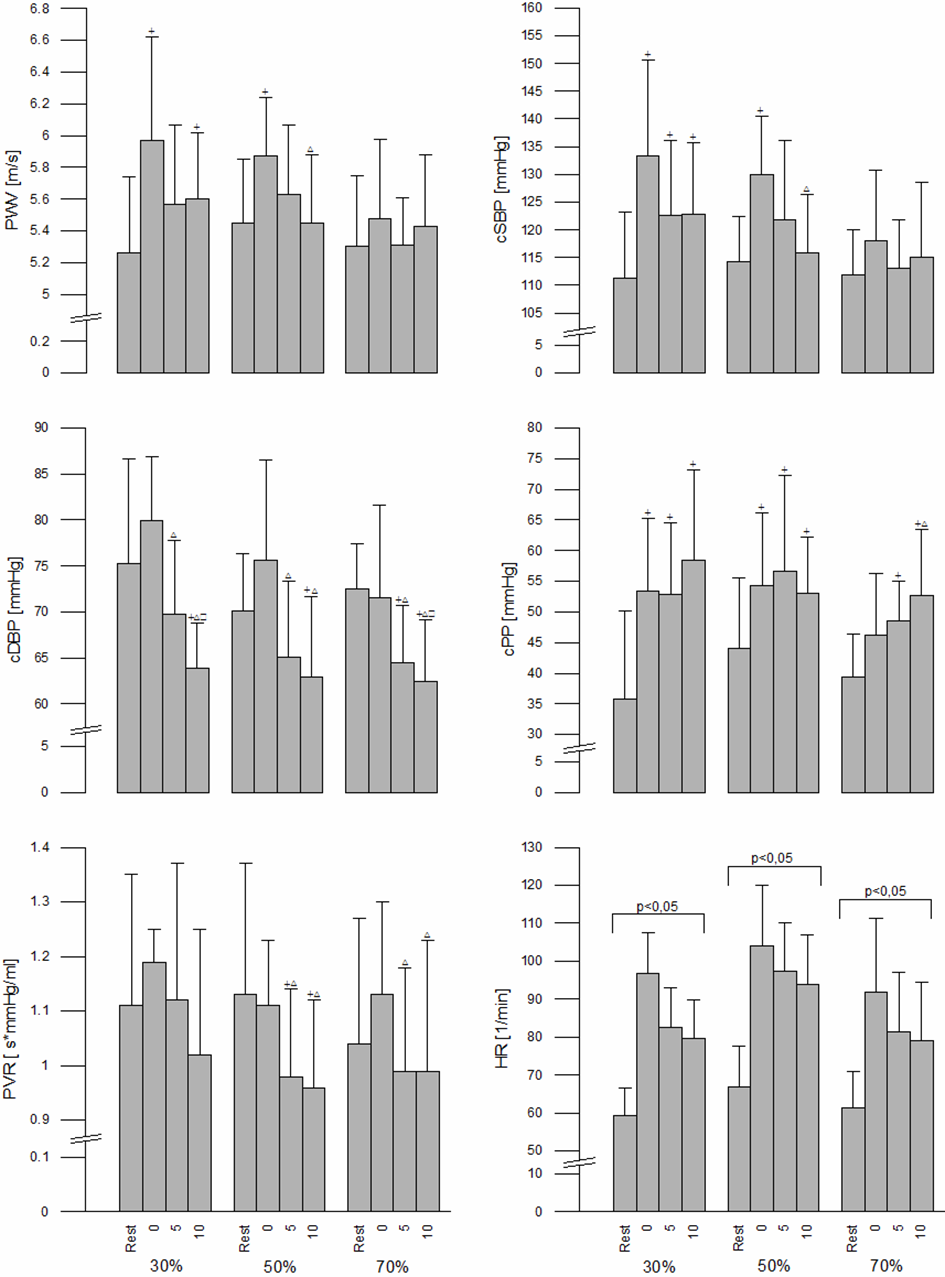 | Figure 2. Changes of the parameters in the different training protocols (+=significant changes from rest, ∆=significant changes to 0 after load, □=significant changes to 5 after load, horizontal bracket indicates significant differences between time points) |
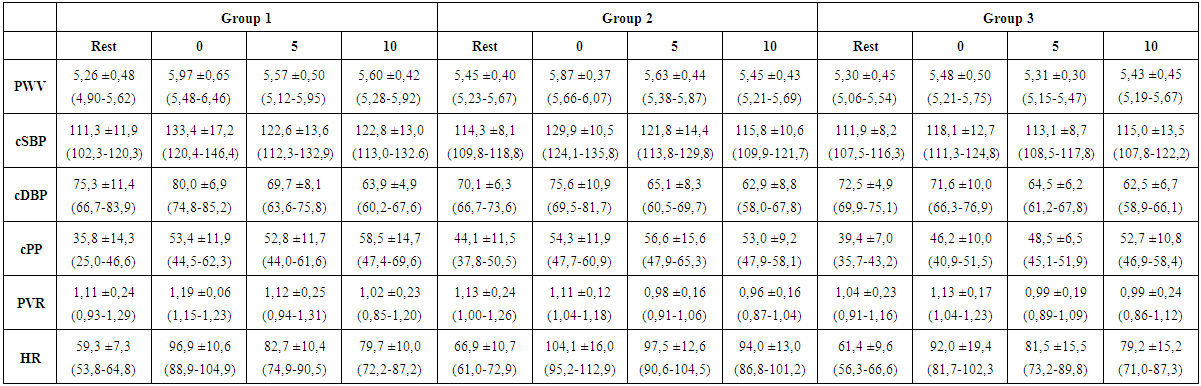 | Table 3. Results (mean ± standard deviation (95% confidence interval)) of measurements in rest, immediately afterload (0), 5 minutes afterload (5) and 10 minutes afterload (10) (PWV=pulse wave velocity in m/s, cSBP=central systolic blood pressure in mm/Hg, cDBP=central diastolic blood pressure in mm/Hg, cPP=central pulse pressure in mm/Hg, PVR=peripheral vascular resistance in s*mmHg/ml, HR=heart rate in 1/min) |
|
4. Discussion
- Arterial stiffness is an important predictor for cardiovascular risk and can be positively influenced by chronic exercise training. So far, there are few studies that examined the acute effects of resistance training on arterial stiffness and central blood pressure values immediately after a training session, e.g. [23]. No study compared different strength training protocols with different load characteristics so far. This study was designed to investigate the acute changes after strength training in typical practice-oriented strength training protocols with different levels of intensity, repetition numbers and set pauses.The blood pressure is regulated via baroreceptors in the aortic arch and carotid sinus, which are stimulated by the stretching of the vascular wall. Here, the brain stem is constantly informed about the pressure conditions in the arterial vascular system and controls the activation and inhibition of the sympathetic and parasympathetic nervous system. A feedback control model describes the regulation of blood pressure. The control variable is the mean arterial blood pressure, which is regulated by cardiac output and PVR [30].Heffernan et al. [18] noted that central arterial stiffness was correlated with baroreflex-sensitivy (BRS) after resistance exercise bout, and changes in central stiffness from rest to recovery were correlated with changes in BRS from rest to recovery. This supports a relationship between BRS and arterial function following acute resistance exercise. Consequently the acute increase of the PWV can be explained with a reduction in BRS following resistance exercise [18]. This could explain the increase of PWV in all three training protocols. The lower increase of PWV at 70% could be explained by a lower impact of this protocol on the BRS.Furthermore, the blood circuit is adapted to physical stress by local chemoreceptors, which are activated by hypoxia, acidosis and hypercapnia, which increases the blood flow and vasodilation in the working muscles [30]. A potential mechanism explaining acute reductions of the cDBP and the peripheral PWV after the bout of exercise is the sheer stress-induced release of nitric oxide (NO) from the endothelium [22]. Assuming that arterial stiffness is dependent on the concentration of NO [31-33], an increased concentration of NO can possibly be found in the vascular system after 70%, because no significant increase in PWV and in cSBP can be shown in this protocol. Guzel et al. [34] has shown that high intensity resistance exercises caused increases in NO and low intensity resistance exercises did not affect NO levels. In a study with chronic heart failure patients, Meyer et al. [35] could not detect any differences between blood pressure increase after strength training with 60% and 80% exercise intensity. However, the PWR after 80% was significantly lower than at 60%. As well as in the present study, the blood pressure increase at higher exercise intensities appeared to be reduced by a decrease in PWR. The increased NO release of the endothelium could play an important role with regard to the arterial stiffness.The slight changes of PWV and cSBP after 70% could be due to the lower number of repetitions and the related lower time under tension in this protocol. This protocol increased the heart rate significantly less. Therefore, a smaller total stress on the cardiovascular system can be concluded, which leads to a lower cardiac output and therefore a smaller increase in blood pressure parameters.After an acute bout of exercise, Collier et al. [36] found that central PWV decreased by 8% after an aerobic exercise and remained at this level for 60 minutes, whereas a whole body resistance exercise increased the central PWV by 9.8% from pre to 40 and 60 minutes after exercise. A comparison of this study with the present one is not sensible, because the measuring time points are different. Heffernan et al. [18] showed 20 minutes after a full-body strength training with 10 repetition maximum (approximately 70% of 1RM) in a comparable collective, a significant increase of PWV of 4.8 ± 0.30 to 5.8 ± 0.31 m/s. Similar results demonstrated Yoon et al. [19] after a full-body strength training with 65% of 1RM. 20 minutes after the exercise, they found a significant increase of PWV compared to the control group, without a significant change of the central blood pressure parameters. However, there was only a PWV increase of approximately 0.3 m/s. In contrast to the studies above, a higher PWV 10 minutes after exercise was observed in this study only at the 30% intensity level. After a full-body strength training with 65% of 1RM (five exercises, three sets, 10 repetitions, 120 s set pause) there were no significant differences between PWV and central blood pressure values after the exercise compared to the control group [21]. This study used three of five exercises for lower extremities. In contrast to that, the present strength training protocol consisted of only one exercise for the lower limbs and this one was the first exercise in the protocol, followed by four upper body exercises.Li et al. [23] showed that strength training with 70% of 1RM for the upper limb increased arterial stiffness immediately after training. However, a strength training with the same intensity for the lower limb and a full body workout decreased the vascular stiffness.The four exercises for the upper extremities were the last four exercises in our training protocol. This could lead to an increased PWV immediately after training. Similarly at Lefferts et al. [37] an increased PWV of 1 m/s for the upper extremity was found 10 minutes after a strength training. Subsequent studies should therefore consider the influence of exercise selection (upper or lower limb) as well.In the same study [37] significant changes of the cSBD and the cDBP 10, 20 and 30 minutes after exercise were shown. The changes 10 minutes after the training could be confirmed by our study.A similar reaction of PWV was found even after intensive sprint loads [38]. In the present study, the load was intensive as well, because the subjects completed the full number of repetitions in every set, if necessary with support from another person.This is the first study that examined various exercise protocols. The five exercises are often used in practice and consist of free exercises with barbells and leaded exercises at a multi-press. Li et al. [23] used only leaded exercises at sequence devices. An analysis with regard to the differentiation between individual exercises can provide additional information about the hemodynamic effects of strength training contents. At this point, a differentiation between exercises for a small and a large percentage of the whole body muscle mass is important, because a different percentage results in a different strain of the cardiorespiratory system [25, 39, 40]. In addition, aspects of the movement frequency need to be considered. Moreover, it can be assumed, that the order of exercises is important. For reasons of standardization, the exercises have been completed in the same order in all three groups in this study. The last exercise was triceps extensions. This may have influenced the acute measurement at the end of the training. Future studies should implement a randomization of the order. Another factor influencing acute changes of PWV is the breathing during exercise execution. Mak et al. [41] showed that the PWV after a strength training with Vasalva-maneuvers increased significantly more than after a strength training with continuous breathing. The measurement method used in our paper does not allow an analysis during dynamic work. The measurement time of the analysis would be too long for practical training protocols. However, we should retain that the extent of the values of the examined parameters do not reflect the exact value during dynamic work, because the measuring time was about 60 seconds. Measurements with a catheter would significantly affect the performance and the motivation of volunteers. Therefore, the employed non-invasive method is a suitable way to quantify the arterial stiffness immediately after physical strain.
5. Conclusions
- The present investigation allows conclusions about physiological effects of different dynamic strength training protocols of active young healthy subjects. The performed protocols showed a physiological adaption of the cardiovascular system. Resistance exercises with low, moderate and high intensity let to a modest increase of PWV, cSBP, cPP and HR. Only those protocols with lower load and more repetitions acutely increased arterial stiffness (PWV, cSBP, cPP) in healthy subjects significantly. The cDBP showed 5 and 10 minutes after a training session a reduction compared to 0 minutes after training in all of the training protocols. For practical conclusions in specific target groups, like older healthy adults or patients with cardiovascular diseases, the protocols need to be tested in these groups.
References
| [1] | Bjarnason-Wehrens B, Mayer-Berger W, Meister ER, Baum K, Hambrecht R, Gielen S. Recommendations for resistance exercise in cardiac rehabilitation. Recommendations of the German Federation for Cardiovascular Prevention and Rehabilitation. European Journal of Cardiovascular Prevention & Rehabilitation 2004; 11(4): 352–61. |
| [2] | Haskell WL, Lee I, Pate RR, Powell KE, Blair SN, Franklin BA et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Medicine and science in sports and exercise 2007; 39(8): 1423–34. |
| [3] | Williams MA, Haskell WL, Ades PA, Amsterdam EA, Bittner V, Franklin BA et al. Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation 2007; 116(5): 572–84. |
| [4] | Carlson DJ, Dieberg G, Hess NC, Millar PJ, Smart NA. Isometric exercise training for blood pressure management: a systematic review and meta-analysis. Mayo Clinic proceedings 2014; 89(3): 327–34. |
| [5] | Cornelissen VA, Fagard RH, Coeckelberghs E, Vanhees L. Impact of resistance training on blood pressure and other cardiovascular risk factors: a meta-analysis of randomized, controlled trials. Hypertension 2011;58(5):950–8. |
| [6] | Baulmann J, C.-P. Herzberg C, Störk T. Die Renaissance von Pulswellengeschwindigkeit, Augmentation und zentralem Aortendruck als Determinanten des kardiovaskulären Risikos. Med Welt 2013(64):30–3. |
| [7] | Baulmann J, Weber T, Mortensen K. Messmethoden der Arteriellen Gefäßsteifigkeit. Austrian Journal of Hypertension 2010; 14(2): 18–24. |
| [8] | Agabiti-Rosei E, Mancia G, O'Rourke MF, Roman MJ, Safar ME, Smulyan H et al. Central blood pressure measurements and antihypertensive therapy: a consensus document. Hypertension 2007; 50(1): 154–60. |
| [9] | Roman MJ, Devereux RB, Kizer JR, Lee ET, Galloway JM, Ali T et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension 2007; 50(1): 197–203. |
| [10] | Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. Journal of the American College of Cardiology 2014; 63(7): 636–46. |
| [11] | Ashor AW, Lara J, Siervo M, Celis-Morales C, Mathers JC. Effects of exercise modalities on arterial stiffness and wave reflection: a systematic review and meta-analysis of randomized controlled trials. PloS one 2014; 9(10):e110034. |
| [12] | Montero D, Roche E, Martinez-Rodriguez A. The impact of aerobic exercise training on arterial stiffness in pre- and hypertensive subjects: a systematic review and meta-analysis. International journal of cardiology 2014; 173(3): 361–8. |
| [13] | Li Y, Hanssen H, Cordes M, Rossmeissl A, Endes S, Schmidt-Trucksäss A. Aerobic, resistance and combined exercise training on arterial stiffness in normotensive and hypertensive adults: A review. European Journal of Sport Science 2014; 15(5): 443–57. |
| [14] | Miyachi M. Effects of resistance training on arterial stiffness: a meta-analysis. Br J Sports Med 2013; 47(6): 393–6. |
| [15] | Rossow LM, Fahs CA, Thiebaud RS, Loenneke JP, Kim D, Mouser JG et al. Arterial stiffness and blood flow adaptations following eight weeks of resistance exercise training in young and older women. Experimental gerontology 2014; 53:48–56. |
| [16] | Okamoto T, Masuhara M, Ikuta K. Effect of low-intensity resistance training on arterial function. Eur. J. Appl. Physiol. 2011; 111(5): 743–8. |
| [17] | DeVan AE, Anton MM, Cook JN, Neidre DB, Cortez-Cooper MY, Tanaka H. Acute effects of resistance exercise on arterial compliance. J. Appl. Physiol. 2005; 98(6):2287–91. |
| [18] | Heffernan KS, Collier SR, Kelly EE, Jae SY, Fernhall B. Arterial stiffness and baroreflex sensitivity following bouts of aerobic and resistance exercise. Int J Sports Med 2007; 28(3): 197–203. |
| [19] | Yoon ES, Jung SJ, Cheun SK, Oh YS, Kim SH, Jae SY. Effects of acute resistance exercise on arterial stiffness in young men. Korean Circ J 2010; 40(1):16–22. |
| [20] | Fahs CA, Heffernan KS, Fernhall B. Hemodynamic and vascular response to resistance exercise with L-arginine. Medicine and science in sports and exercise 2009; 41(4): 773–9. |
| [21] | Thiebaud RS, Fahs CA, Rossow LM, Loenneke JP, Kim D, Mouser JG et al. Effects of age on arterial stiffness and central blood pressure after an acute bout of resistance exercise. Eur J Appl Physiol. 2016;116(1):39-48. |
| [22] | Heffernan KS, Rossow L, Jae SY, Shokunbi HG, Gibson EM, Fernhall B. Effect of single-leg resistance exercise on regional arterial stiffness. Eur. J. Appl. Physiol. 2006; 98(2): 185–90. |
| [23] | Li Y, Bopp M, Botta F, Nussbaumer M, Schäfer J, Roth R et al. Lower Body vs. Upper Body Resistance Training and Arterial Stiffness in Young Men. International journal of sports medicine 2015; 36(12): 960–7. |
| [24] | Nitzsche, N., Groß, P., Weigert, M., Schulz, H. Gefäßsteifigkeit bei unterschiedlichen isometrischen Muskelspannungen. In: Granacher U, editor. Krafttraining - kraftvoll durchs Leben, 98. |
| [25] | Fleck SJ, Kraemer WJ. Designing resistance training programs. 4th ed. Champaign, Ill.: Human Kinetics; 2014. |
| [26] | Kraemer WJ, Fleck SJ. Optimizing strength training: Designing nonlinear periodization workouts. Champaign, IL: Human Kinetics; 2007. |
| [27] | Franssen PM, Imholz BP. Evaluation of the Mobil-O-Graph new generation ABPM device using the ESH criteria. Blood pressure monitoring 2010; 15(4): 229–31. |
| [28] | Hametner B, Wassertheurer S, Kropf J, Mayer C, Eber B, Weber T. Oscillometric estimation of aortic pulse wave velocity: comparison with intra-aortic catheter measurements. Blood pressure monitoring 2013;18(3):173–6. |
| [29] | Feistritzer H, Reinstadler SJ, Klug G, Kremser C, Seidner B, Esterhammer R et al. Comparison of an oscillometric method with cardiac magnetic resonance for the analysis of aortic pulse wave velocity. PloS one 2015;10(1):e0116862. |
| [30] | Mutschler E, Schaible H, Vaupel P, Thews G, Thews-Mutschler-Vaupel. Anatomie, Physiologie, Pathophysiologie des Menschen: 140 Tabellen. 6th ed. Stuttgart: Wiss. Verl.-Ges; 2007. |
| [31] | Cocks M, Wagenmakers AJM. The effect of different training modes on skeletal muscle microvascular density and endothelial enzymes controlling NO availability. J Physiol. 2016 Apr 15; 594(8):2245-57. |
| [32] | Green DJ, Maiorana A, O'Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. The Journal of physiology 2004; 561 (Pt 1): 1–25. |
| [33] | Nosarev AV, Smagliy LV, Anfinogenova Y, Popov SV, Kapilevich LV. Exercise and NO production: relevance and implications in the cardiopulmonary system. Frontiers in cell and developmental biology 2014; 2: 73. |
| [34] | Guzel NA, Hazar S, Erbas D. Effects of different resistance exercise protocols on nitric oxide, lipid peroxidation and creatine kinase activity in sedentary males. Journal of sports science & medicine 2007; 6(4): 417–22. |
| [35] | Meyer K, Hajric R, Westbrook S, Haag-Wildi S, Holtkamp R, Leyk D et al. Hemodynamic responses during leg press exercise in patients with chronic congestive heart failure. The American Journal of Cardiology 1999; 83(11): 1537–43. |
| [36] | Collier SR, Diggle MD, Heffernan KS, Kelly EE, Tobin MM, Fernhall B. Changes in arterial distensibility and flow-mediated dilation after acute resistance vs. aerobic exercise. Journal of strength and conditioning research / National Strength & Conditioning Association 2010; 24(10): 2846–52. |
| [37] | Lefferts WK, Augustine JA, Heffernan KS. Effect of acute resistance exercise on carotid artery stiffness and cerebral blood flow pulsatility. Front. Physiol. 2014; 5(782):740. |
| [38] | Rakobowchuk M, Stuckey MI, Millar PJ, Gurr L, Macdonald MJ. Effect of acute sprint interval exercise on central and peripheral artery distensibility in young healthy males. Eur. J. Appl. Physiol. 2009; 105(5):787–95. |
| [39] | Åstrand P. Textbook of work physiology: Physiological bases of exercise. 4th ed. Champaign, IL: Human Kinetics; 2003. |
| [40] | Wilson JR, Sharples S. Evaluation of human work. Boca Raton, FL: CRC Press, Taylor & Francis Group; 2015. |
| [41] | Mak WYV, Lai WKC. Acute Effect on Arterial Stiffness after Performing Resistance Exercise by Using the Valsalva Manoeuvre during Exertion. BioMed research international 2015; 2015: 343916. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML