-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Sports Science
p-ISSN: 2169-8759 e-ISSN: 2169-8791
2016; 6(2): 52-61
doi:10.5923/j.sports.20160602.06

Cardiac Remodeling and Physical Exercise: A Brief Review about Concepts and Adaptations
Paula A. M. Cavalcante1, Mauro S. Perilhão1, Ariana A. da Silva1, Andrey J. Serra2, Aylton F. Júnior1, Danilo S. Bocalini1, 3
1Translational Physiology Laboratory, Postgraduate Program in Physical Education, São Judas Tadeu University (USJT), São Paulo, SP, Brazil
2Postgraduate Program in Biophotonics Applied to Health Sciences, Nove de Julho University (UNINOVE), São Paulo, SP, Brazil
3Translational Physiology Laboratory, Aging Science, São Judas Tadeu University (USJT), São Paulo, SP, Brazil
Correspondence to: Danilo S. Bocalini, Translational Physiology Laboratory, Postgraduate Program in Physical Education, São Judas Tadeu University (USJT), São Paulo, SP, Brazil.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Training variable control, is relateed to frequency, duration, and intensity, that respond to the promoting changes in physical fitness. However, adaptations related to different exercise intensities are still controversial, both in regards to cardiovascular disorder prevention and rehabilitation. There are few studies dealing with cardiac adaptations under different exercise intensities, and that a better understanding physiological cardiac remodeling may inspire the design of a strategy to improve ventricular function in cardiac approach. Thus the objective of this review was to assess the influence of chronic aerobic exercise on cardiac adaptations, with a focus on different exercise intensities, as well as presenting a conceptual approach on cardiac remodeling process. Also was included the specific characteristics of cardiac remodeling in each cardiac adequacy step during training. New research trends were also outlined in order to guide studies into developing new therapy strategies for controlling cardiovascular diseases.
Keywords: Cardiac remodeling, Physical exercise, Cardiac adaptations and ventricular function
Cite this paper: Paula A. M. Cavalcante, Mauro S. Perilhão, Ariana A. da Silva, Andrey J. Serra, Aylton F. Júnior, Danilo S. Bocalini, Cardiac Remodeling and Physical Exercise: A Brief Review about Concepts and Adaptations, International Journal of Sports Science, Vol. 6 No. 2, 2016, pp. 52-61. doi: 10.5923/j.sports.20160602.06.
Article Outline
1. Introduction
- The chronic effect of aerobic exercise has been studied in order to further and effectively understand the adaptations caused in humans. Based on this perspective, the actions of regular physical exercise and improvements of functional capacity are important factors in improving health. Their beneficial effects on the cardio circulatory system are well described in literature [1-8].However, in relation to exercise intensity on biological and clinical effects, the guidelines is controversial both in cardiovascular disease prevention and in the rehabilitation field [9,10]. Although increased training intensity may result in further improving physical aptitude and maximum oxygen consumption (VO2max) [2], moderate intensity exercise is usually recommended, as it is equally efficient [9].This literature review assesses the influence of chronic aerobic exercise on cardiac adaptations considering there are few studies in literature regarding cardiac adaptations in different exercise intensities, and that a better understanding of the physiological cardiac remodeling may lead to a strategy capable of improving ventricular function in cardiac dysfunction [11, 12]. In particular, this review will focus on the discussion regarding the different exercise intensities, presenting a conceptual approach on the cardiac remodeling process and its particular characteristics in each cardiac adaptation step for physical training. New research trends were also outlined in order to guide studies into developing new therapeutic strategies for controlling cardiovascular diseases.
2. Main Body
- The most relevant original scientific studies (animals and human) were from 1979 to 2015, analyzed in this review, at the following databases: Science Citation, Index, Scopus, Sport Discus, Scielo, and National Library of Medicine, combining the following: keywords: endurance training, physical exercise, cardiac function, ventricular function, cardiac remodeling, ventricular remodeling and cardiac and ventricular adaptations. Studies investigating the effects of physical exercise and cardiac parameters with and training variables (as well as human and animal studies) were considered inclusion criteria.
3. Cardiac Remodeling
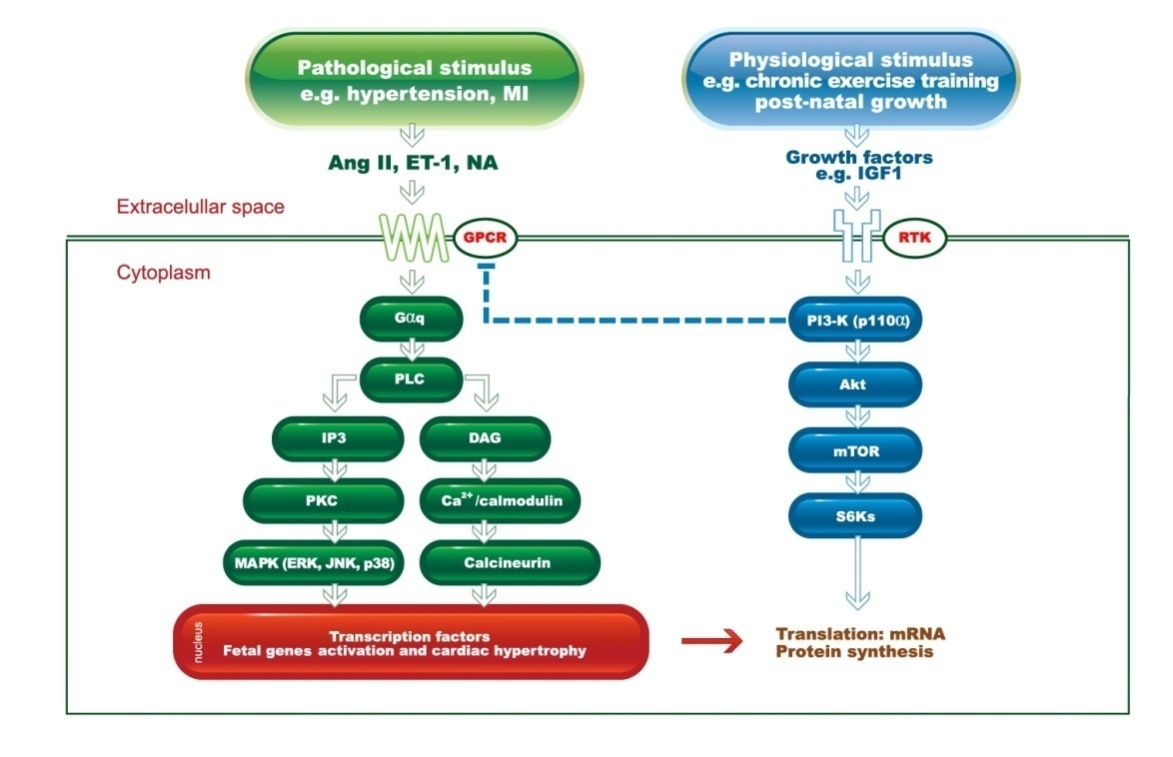
- Cardiac remodeling (CR) is a group of genetic, molecular, cellular, and interstitial changes in the myocardium which are anatomically manifested by changes in mass, size, and geometry of the heart chambers and function stemming from hemodynamic overload [74-76].Recent studies [77-79] suggest epigenetic changes in the DNA, resulting from physical exercise, can also change the functions of the cardiovascular system, although there is insufficient evidence to establish a direct link between epigenetic modulations and changes, caused by exercise, in the heart and blood vessels [78]. This likely occurs due to the cardiovascular field of research not being nearly as advanced as others in investigating epigenetics [77]. Nevertheless, evidence reveal physical exercise is capable of neutralizing the development of pathological epigenetics in diseases such as hypertension, atherosclerosis, and fibrosis [79]. As such, it is possible that epigenetic changes may also be mentioned as part of the concept of cardiac remodeling in the near future.Following an analysis of new trends, a recent study [80] showed that it is possible to reduce cardiac hypertrophy causing blockage of TLR4 in the brain in mice suffering from arterial hypertension induced by angiotensin II. The actions of receptor toll-like 4 (TLR4) are associated with the development and progression of cardiovascular diseases [81]. Dange et al [80] proved there could be a bidirectional link between the brain suffering from stress and pathological cardiac hypertrophy. Researchers speculated that the toll-like receptors’ activity in the brain, particularly TLR4, is key in structure modulation and cardiac function.Characterized as end product of several types of hemodynamic stimulus, the CR may be called physiological during normal development, or in athletes [75], or pathological, common to multiple aggressions to the heart. The aggressions can be injury (myocardial infarction), chronic pressure overload (hypertension, aortic stenosis) or volume (aortic or mitral insufficiency), inflammation (myocarditis), or expression of a genetic program leading to cardiomyopathy [82]. These events allow the heart to adapt to new conditions and activate important mediators including wall stress, neuro hormones (sympathetic nervous system, renin-angiotensin, aldosterone and endothelin), cytokines, nitric oxide and oxidative stress [75].The main adaptation of the heart to physical training is the CR [24, 69, 83, 84], and the cardiac adaptations are related to structural and functional cardiac changes, influenced by the gain of functional ability acquired in training. The major physiological mechanisms attributed to the CR is increased contractility [24, 85-87], improved transient Ca2+ intracellular affecting ventricular function [87-89], increased expansion with significant improvements in myocardial oxygenation [90], and additional endothelium - dependent functions that prevent ischemic events [9]. However, CR’s physiological answers are not uniform and vary according to the type and intensity of the exercise performed, as well as the physical training program [91] and is directly related to maximal aerobic capacity or VO2 max [92, 93].The beginning of the CR process is driven by a hemodynamic stimulus from a transient overload, such as what occurs in growth and physical training or a persistent overload arising from events such as myocardial infarction or from some diseases such as hypertension, as shown in Figure 1. In this perspective, CR results may have different consequences and outcomes according to the type and the time of the stimulus generator [12, 94]. Thus, the CR, considering one of the most significant results of chronic adaptation to exercise, derives in myocyte hypertrophy in cardiac performance to suit the demands of the body [95, 96].
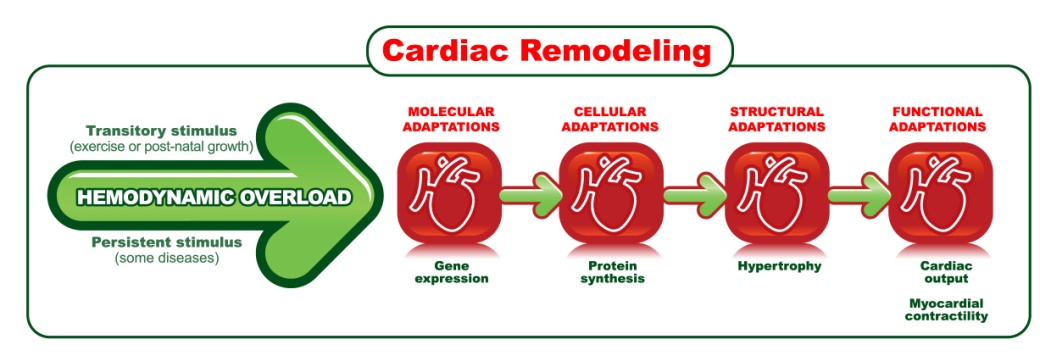 | Figure 1. Steps of cardiac remodeling |
4. Cardiac Adaptation in Chronic Aerobic Exercise
- Effects of modalityWithin the exercise protocols that were used, positive effects of chronic aerobic training both in humans [2, 11, 13-16] and animals [9, 17, 18] were shown to improve ventricular function, with particular increase in cardiac output and stroke volume. Other studies have also assessed performance in isolated hearts [19, 20], papillary muscles [21, 22], and isolated myocytes [9, 23, 24]. All of these studies highlighted improvements in cardiac function.Another aspect to consider is the different in magnitude of cardiac hypertrophy, which is directly related to the training protocol that was used [25-28]. In this regard, swimming is frequently used in exercise physiology studies on mice, as swimming is an innate ability [29, 30] with decreased costs compared to using treadmills. Furthermore, studies using this model detected similarities in adapting to exercise, particularly in regards to what was observed in humans [27, 30].Thus, swimming training in mice is recognized for its efficient introduction of robust cardiac hypertrophy compared to exercising on treadmills [18, 27], promoting significant increase in the end diastolic volume of the left ventricle [18, 31]. While following this line of thinking, Schable & Scheuer (1981) [18] showed that chronic swimming training in mice led to improvements of the left ventricle’s contractile performance, although there was a notable presence of hypertrophy compared to treadmill running, in which they were not observed or hypertrophy or improved function.Indeed, most studies of treadmill running were unable to observe cardiac hypertrophy in mice [18, 32, 33], but some researchers noticed its presence in trained mice under running protocols [34, 35]. In both of Kemi’s (2002, 2007) [34, 36] studies involving high intensity training on treadmill, there was a significant increase in the size of cardiomyocytes, and both contractility and cardiac function were reinforced. This suggests the high intensity in treadmill or wheel-training programs also appears to be effective in inducing physiological hypertrophy [27].Effects of intensityNot much is known from the standpoint of adaptations related to different intensities. In order to reduce the cardiovascular risk factors, the exercise intensity estimate calculated from reserve heart rate usually fluctuates between 60 and 80% [37]. However, professionals and conservative institutions usually prescribe intensity of aerobic exercise for adults and the elderly, regardless of whether or not they are at risk of cardiovascular disease, based on the ventilator threshold offered by ergospirometry. For individuals suffering from cardiac insufficiency, prescription of the upper limit is established to be 10% lower than the value registered on the respiratory compensation point, thus preventing exercise from being carried out with decompensated metabolic acidosis [38].Although high intensity exercise is generally avoided due to the risks on cardiac disease patients, Rognmo et al. [2], in 2012, revealed that the adverse event rate associated with this kind of training is low. Therefore, it can be considered adequate for patients with coronary arterial disease. In fact, several studies [1, 2, 11, 14, 39, 40] showed increased effectiveness in obtaining physical capacity, quality of life, and risk factor control when the population was submitted to higher intensity training, showing the importance and safety of increased intensity. These findings may change certain paradigms in the future [38].It is also known that vigorous exercise may greatly and progressively increase of sudden cardiac death and myocardium failure in susceptible individuals [41]. In this analysis, the ideal dosage of high intensity training is still perceived as a subject of research. It has already been shown that a single weekly session of high intensity exercise can reduce the risks of cardiovascular death in male and female with no records of such diseases [42] and in patients with established coronary disease [43].The recommendations presented by ACSM and AHA [44] related to chronic diseases prevention in adults and the elderly includes a 5-day routine with 30 minute sessions of moderate-vigorous aerobic activity, or a 3-day routine with 20 minute sessions of vigorous activity or a combination of both intensities. These recommendations also suggest that an increase of aerobic activity dose may be necessary for some groups that attempt to prevent the transition from excessive weight or obesity [45]. Still, there is no prescription of possible exercise prevention excess related to diseases. However, it appears that it is suggested, at least from a public health perspective, to restrict vigorous physical training to 60 minutes a day, with the maximum hours per week not exceeding 5 hours, including 1 or 2 days off from high intensity exercises [45, 46].In fact, excessive exercise may be cardiotoxic in some responsiveness individuals [47-49] and following this approach, there is a relationship between the accumulated hours of exercise and the risk of atrial fibrillation [47]. Studies on mice, without pathogenic agents [50] and in marathon athletes [51] who underwent excessive training throughout the years revealed an association with myocardium inflammation and fibrosis. This leads to a substrate for arrhythmogenesis and myocardium dysfunction. However, the subjacent mechanisms have yet to be cleared up, although the atrial and structural changes, including dilatation and fibrosis, are present [47].Additionally, recent studies [52, 53] suggest that long distance runners may show, throughout years of uninterrupted training, increased levels of atherosclerosis and coronary diseases. Excessively high doses of resistance exercise in certain genetically predisposed individuals or with pre-existing diseases [48, 49] may lead to dilatation and cardiac dysfunction [46], particularly on the right side of the heart and the ventricular septum, which conducts the release of cardiac markers, such as troponin and cerebral natriuretic peptide. This results in malignant ventricular arrhythmia and increased risk of sudden cardiac death [46]. Even so, there are still certain segments of the literature dealing with themes capable of establishing the limit of exercise for potential cardiac toxicity, tracking individuals at risk, and designing ideal physical training programs to optimize cardiovascular health [45].It is for this reason that the evidence that many years of high intensity training may lead to cardiac fibrosis or acceleration of atherosclerosis may appear to be relatively weak, considering there is no definitive argument that excessive endurance training may likely be harmful for the human heart [46]. Finally, evidences may lead to studies [54, 55], signaling that excessively high levels of endurance training may prevent the decrease of complacence and stretching of blood vessels, as seen in the healthy aging.The importance of exercise intensity was shown by studies on humans [2, 11, 15, 56] and mice undergoing treadmill training [9, 24, 34]. These studies demonstrated that high intensity physical aerobic exercise may be more favorable for adaptations in cardiorespiratory fitness and cardiac function, compared to low and moderate intensity exercise, further suggesting that vascular and peripheral changes may contribute to the effects which depend on intensity, such arterial endothelial function and its regulating mechanisms [17]. From this standpoint, the study conducted by [9] on mice using treadmills, without pathogenic agents, showed that cardiovascular adaptations to training depend on intensity. The close correlation among VO2max, the dimensions of cardiomyocytes, and contractile capability suggested that there is a significant benefit under high intensity compared to the effects on endothelial function caused by regular exercise under moderate intensity [9].With this aspect in mind, epidemiological and clinical approaches have shown that physical exercise adaptations may depend of both on intensity and total volume of training [15, 16, 57-60]. Such fact may be associated with the metabolic equivalent or maximum consumption of oxygen. In this analysis, it is likely that the oxygen transport system undergoes beneficial adaptation, which becomes more evident through higher VO2max values as a result of function adaptations stemming from the cardiac remodeling process, as caused by aerobic physical training [61]. Thus, peak oxygen consumption (VO2peak) has shown as an important clinical reference, having been identified as one of the greatest independent predicting agents of mortality, both in healthy individuals and those susceptible to cardiac diseases [37, 57, 62, 63, 64].However, despite the importance of this marker, upon analyzing the general recommendations both for prevention and rehabilitation, literature reveals intensity variations corresponding between 40% to 85% of the VO2max [65, 66], causing controversies regarding the biological and clinical effects between moderate and high intensity exercises [9, 10, 67]. As such, from a physical aptitude standpoint, intensity variation differs between the VO2max gains both in humans [13, 68] and in animals [9, 24, 36, 69, 70, 71], be them healthy [9, 11, 24, 36, 68-73] or at risk of cardiac diseases [13, 15, 16], reiterating the idea that the adaptations are conditioned to exercise intensity.
5. Conclusions
- Studies confirm the myocardial adaptations are closely related to the gain of VO2 max. However, much effort is still needed to assess the effects of chronic intensity of aerobic exercise in the cardiovascular system, especially at the molecular level, to find out strategies to prevent and treat cardiovascular diseases. In this context, it is necessary to further studies related to the effects of physical exercise in epigenetic modulation, as this type of intervention may be an interesting strategy in the cardiovascular field.The integration of studies involving the responses of the immune system in the brain and heart seems to be a trend in a near future. These findings may help develop new therapeutic strategies to control cardiovascular diseases. Future researches will contribute to better understanding if longitudinal follow-up in large groups of endurance athletes (e.g. marathon runners), in order to assess and determine the exercise limit for potential cardiac toxicity, considering the tracking of individuals at risk.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML