-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Sports Science
p-ISSN: 2169-8759 e-ISSN: 2169-8791
2015; 5(6): 234-239
doi:10.5923/j.sports.20150506.03

Acute Changes in Soluble Cell Adhesion Molecules Following Different Intensities of Resistance Exercise
Jin K. Park1, Neil Schwarz2, Darryn Willoughby1, Yunsuk Koh1
1Department of Health, Human Performance, and Recreation, Baylor University, Waco, USA
2Department of Health, Physical Education, and Leisure Studies, University of South Alabama, Mobile, USA
Correspondence to: Yunsuk Koh, Department of Health, Human Performance, and Recreation, Baylor University, Waco, USA.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The current study examined the effects of lower- and higher-intensity resistance exercise on acute changes in soluble cell adhesion molecules. In a cross-over design, 10 recreationally-trained college-aged men performed resistance exercises at two different intensities in random order (lower-intensity: 50% of 1-RM and higher-intensity: 80% of 1-RM). Overnight fasting blood samples were analysed for soluble intracellular adhesion molecule-1 (sICAM-1) and soluble vascular adhesion molecule-1 (sVCAM-1) at baseline, 3-hr, 24-hr, and 48-hr post exercise (PE). sICAM-1 at 48-hr PE (364.85 ± 34.40 ng/ml) significantly (p = .037) decreased from 3-hr PE (425.60 ± 36.71 ng/ml) during the higher-intensity exercise trial. sVCAM-1 at 24-hr following the lower-intensity exercise trial (715.70 ± 38.14 ng/ml) significantly (p = .001) increased by 24% from baseline and remained elevated up to 48-hr PE (716.32 ± 34.79 ng/ml). The current study suggests that higher-intensity resistance exercise does not negatively affect cell adhesion molecules in recreationally-trained men, but it may help improve cardiovascular health by decreasing sICAM-1 without altering sVCAM-1.
Keywords: ICAM-1, VCAM-1, Atherosclerosis, Vascular inflammation
Cite this paper: Jin K. Park, Neil Schwarz, Darryn Willoughby, Yunsuk Koh, Acute Changes in Soluble Cell Adhesion Molecules Following Different Intensities of Resistance Exercise, International Journal of Sports Science, Vol. 5 No. 6, 2015, pp. 234-239. doi: 10.5923/j.sports.20150506.03.
Article Outline
1. Introduction
- Atherosclerosis, the most common form of coronary artery disease (CAD), is strongly associated with dyslipidemia [1-3], endothelial dysfunction, and inflammation [4]. Increased oxidative stress and leukocytes activate the expression of cell adhesion molecules on endothelia, such as intracellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), and lead to endothelial dysfunction and inflammation. An increase in endothelial expression of ICAM-1 and VCAM-1is considered the initial step of atherosclerosis since it facilitates the migration of leukocytes into the intima [5, 6], causing the differentiation of leukocytes to macrophages [7]. Fat-laden macrophages eventually become foam cells and form the fatty streaks of the plaques in the intima [8]. Both ICAM-1 and VCAM-1 can be found in circulation in soluble form, called soluble ICAM-1 (sICAM-1) and soluble VCAM-1 (sVCAM-1) [9, 10], and are released from activated endothelia as a result of inflammation [11]. The levels of sICAM-1 and sVCAM-1 in the circulation are highly correlated with those measured in the endothelium [12]. Thus, circulating levels of sICAM-1 and sVCAM-1 can be considered as potential markers for atherosclerosis and inflammation. A strong body of evidence suggests that exercise in general can be of great benefit to the prevention of atherosclerosis by positively altering blood lipid profiles as well as sICAM-1 and sVCAM-1 [13-15]. However, the effects of exercise performed at different intensities on cell adhesion molecules [16-18] are equivocal. Some previous studies have reported that high-intensity exercise [16, 18], but not low-to moderate intensity exercise [17], may negatively influence cardiovascular health by increasing both sICAM-1 and sVCAM-1. In contrast, several studies have suggested that high-intensity exercise may provide beneficial effects on endothelial function and cardiovascular health without significantly affecting sICAM-1 or sVCAM-1 [19-21]. Both aerobic and resistance exercises have been widely recommended as the non-pharmacological approach to improving cardiovascular health [22]. However, a majority of previous studies that examined the effect of exercise on cardiovascular health have focused primarily on aerobic exercise. Only limited information regarding the effects of resistance exercise on sICAM-1 and sVCAM-1 is currently available. Moreover, according to the previous studies, the responses of cell adhesion molecules following resistance exercise are inconsistent. A few studies have reported a significant change in either sICAM-1 or sVCAM-1 [23, 24], whereas some have not [19, 21]. Additionally, based on our search, no study has yet examined the acute responses of sICAM-1 and sVCM-1 over 48 hours following resistance exercise. Therefore, the current study examined the effects of resistance exercise at different intensities (lower vs. higher) on sICAM-1 and sVCAM-1 in recreationally-trained men.
2. Material & Methods
2.1. Participants
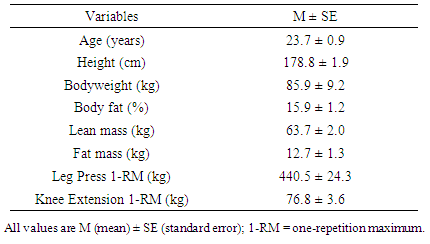
- Ten apparently healthy college-aged men who had resistance-trained 3 to 6 days per week for at least one year prior to the onset of the study volunteered in the study. All participants were at a low risk for cardiovascular disease and had no contraindications to exercise as outlined by the American College of Sports Medicine (ACSM) [25]. All participants had not taken any pharmaceutical or dietary supplement to treat dyslipidemia at least for three months prior to the study and refrained from any exercise during the study period. The participants were asked not to change their diet during the study period and encouraged to consume the same dinner the night before each blood draw. The study protocol was reviewed and approved by the Institutional Review Board for Human Subjects. Informed consent was obtained from all individual participants included in the study.
2.2. Study Design and Exercise Trials
- Using a randomized, cross-over design, the participants undertook three study sessions. During the first entry session, the participants’ anthropometric characteristics and one repetition maximum (1-RM) were assessed. After the entry session, each participant performed two exercise trials in random order (lower-intensity: 50% of 1-RM and higher-intensity: 80% of 1-RM) (Figure 1). Each exercise trial was separated by 7 to 10 days to allow the participants full recovery. Each exercise trial was similar in volume and composed of the leg press and knee extension exercises. During the lower-intensity resistance exercise trial, the participants performed the leg press and unilateral knee extension exercises for 3 sets of 16 repetitions at 50% of 1-RM. During the higher-intensity resistance exercise trial, the participants performed the same lower-body resistance exercises for 6 sets of 5 repetitions at 80% of 1-RM. For both exercise trials, 150 seconds of rest separated sets and exercises. The participants refrained from any types of physical activity until completing the last blood draw at 48-hr following each exercise trial.
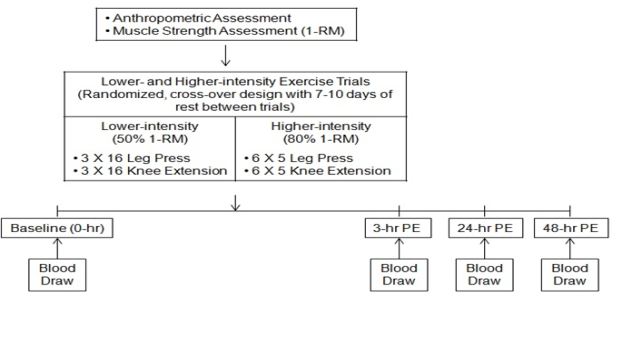 | Figure 1. Study design. RM, repetition maximum; PE; post-exercise |
2.3. One Repetition Maximum Assessment
- Assessment of 1-RM was performed for the leg press and unilateral knee extension during the entry session. The participants warmed-up by completing 5 to 10 repetitions at approximately 50% of their previously known 1-RM, which was based on individual assessment. The participants rested for 1 minute, and then completed 3 to 5 repetitions at approximately 70% of the previously known 1-RM for each exercise. If successfully lifted the weight, the participants rested for 2 minutes before attempting the next weight increment (2.5 – 5.0 kg). This procedure was continued until the participants failed to complete the lift. The 1-RM was recorded as the maximum weight that the participants were able to lift for one repetition. Leg press strength was assessed using an isotonic hip/leg sled (Nebula Fitness, Inc., Versailles, USA), and knee extension strength was assessed using a modular isotonic leg extension machine (Cybex International, Inc., Medway, USA).
2.4. Blood Sample Collection
- Overnight fasting blood samples were collected at baseline (prior to exercise), 3-hr, 24-hr, and 48-hr post-exercise (PE) for each exercise trial. After 10-minutes of resting in a chair, venous blood from the antecubital vein was collected into a serum separator tube. Immediately after blood draw, blood samples remained at room temperature for 20 minutes to be clotted and then were centrifuged (1,000g) for 20 minutes to separate serum. Serum samples were allocated into 1.5 mL tubes and immediately frozen at -80°C for the further analyses.
2.5. Measurements of sICAM-1 and sVCAM-1
- Serum samples in duplicate were analysed for sICAM-1 (Kit# EK0370, BOSTER, Fremont, USA) and sVCAM-1 (Kit# EK0537, BOSTER, Fremont, USA) by an enzyme-linked immunosorbent assay. Optical density was measured at 450 nm using a microplate spectrophotometer (SmartSpec Plus, Bio-Rad, Hercules, USA). Each assay was performed as instructed by the manufacturer’s assay procedure. Inter-assay CV% for sICAM-1 and sVCAM-1 were 5.8% and 2.5%, respectively, and intra-assay CV% for sICAM-1 and sVCAM-1 were 6.4% and 2.7%, respectively.
2.6. Statistical Analyses
- All statistical analyses were performed using the IBM Statistical Package for the Social Sciences 20.0 (IBM SPSS, Armonk, USA) and reported as mean ± standard error (SE) unless stated otherwise. A 2 X 4 [intensity (lower- and higher-intensity) X time (baseline, 3-hr PE, 24-hr PE and 48-hr PE)] analysis of variance (ANOVA) with repeated measures was used to examine the differences in intensity and time on sICAM-1 and sVCAM-1. The lease significant difference (LSD) tests were conducted as post hoc tests to locate the significant mean differences. If a significant interaction between intensity and time was found, the follow-up simple effects test was conducted. A p-value < .05 was set for the statistical significance.
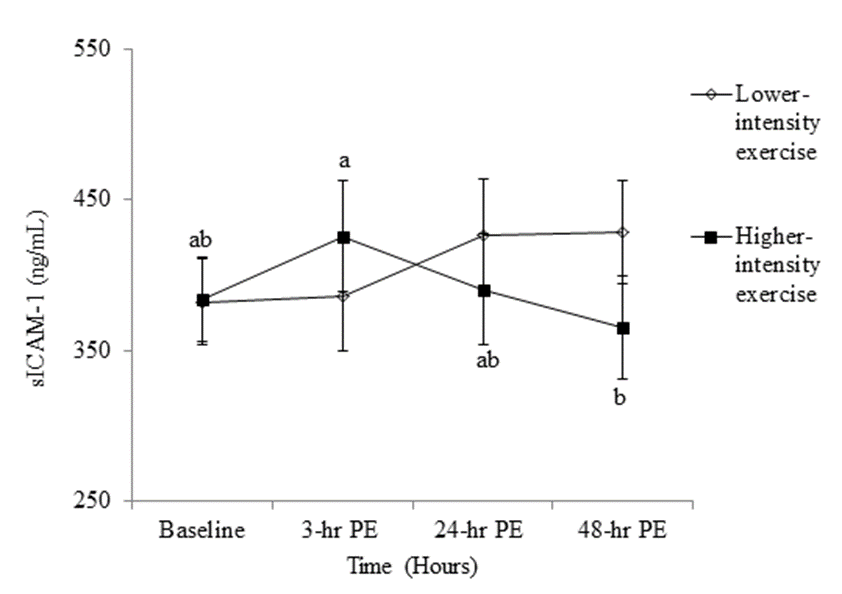
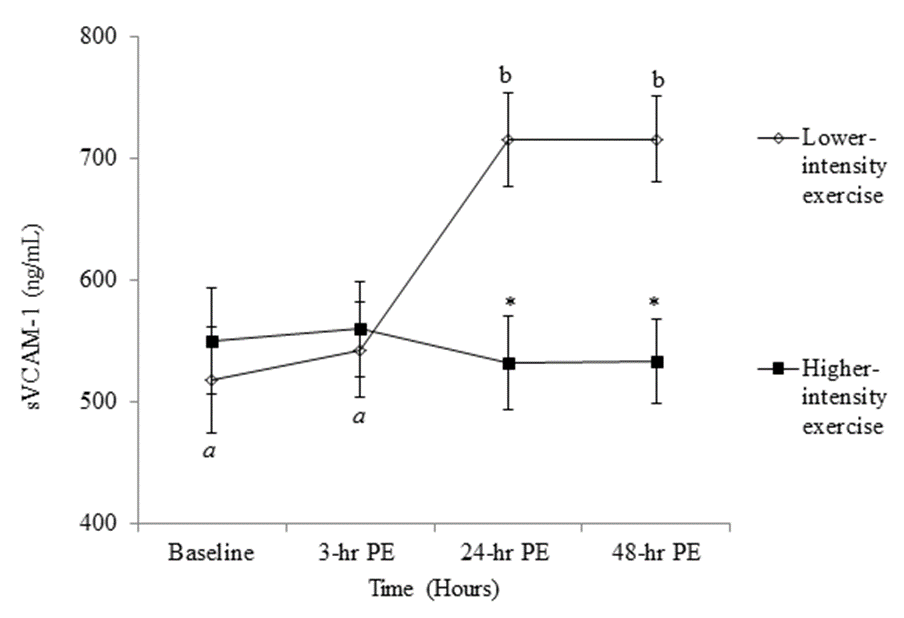
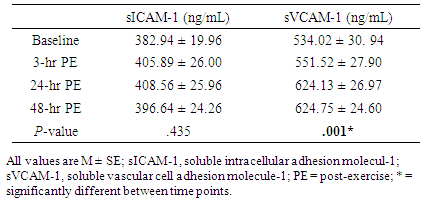
3. Results
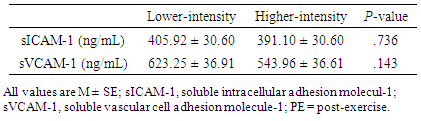
- The anthropometric characteristics including 1-RM of the participants are reported in Table 1. There was no significant main effect for either intensity or time for sICAM-1 (Tables 2 and 3), while the significant interaction between intensity and time was found (p = .046) in sICAM-1. The follow-up simple effects test indicated that sICAM-1 at 48-hr PE (364.85 ± 34.40 ng/mL) significantly (p = .037) decreased by 14.27% from 3-hr PE (425.60 ± 36.71 ng/mL) only during the higher-intensity exercise trial (Figure 2). There was no significant main effect for intensity in sVCAM-1 (Table 3), whereas the main effect for time (p = .001) and the interaction (p = .001) were significant (Table 2). The follow-up simple effects tests indicated that following the lower-intensity exercise trial, sVCAM-1 significantly (p = .001) increased by 27.6% at 24-hr PE (715.70 ± 38.14 ng/mL) and remained elevated up to 48-hr PE (716.32 ± 34.79 ng/mL) (p = .001). In addition, sVCAM-1 at 24-hr (715.70 ± 38.14 ng/mL) and 48-hr PE (716.32 ± 34.79 ng/mL) during the lower-intensity exercise trial were significantly higher (p = .003 and .002, respectively) than the same time points (24-hr PE: 532.57 ± 38.14 ng/mL and 48-hr PE: 533.18 ± 34.79 ng/mL) of the higher-intensity exercise trial (Figure 3).
|
|
|
4. Discussion
- One of the important findings of the current study is that higher-intensity resistance exercise decreased sICAM-1 without altering sVCAM-1, while lower-intensity resistance increased sVCAM-1. Despite the fact that only limited information regarding the effects of resistance exercise on acute changes in sICAM-1 and sVCAM-1 is currently available, the result of the current study was similar to other previous studies [10, 26, 27]. For instance, chronic resistance training at moderate- or high-intensity did not change sVCAM-1 or sICAM-1 in untrained men [27] or women [26], while acute endurance exercise at moderate-intensity [10] and long-distance running exercise in trained men and women [28] increased sICAM-1 and sVCAM-1. In another study, healthy adolescent boys significantly increased sICAM-1 following intense wrestling exercise [29]. These inconsistent results of exercise-induced changes in sICAM-1 or sVCAM-1 may be due to several factors, including different study samples and exercise mode and duration. Additionally, only a few studies have reported the effect of high-intensity resistance exercise on sICAM-1 and sVCAM-1, and have shown that high-intensity resistance exercise did not affect sICAM-1 or sVCAM-1 [19, 21]. The exercise volume used in the aforementioned studies was apparently lower than that was given in the current study. Thus, the exercise volume achieved in the previous studies was not large enough to significantly alter sICAM-1 or sVCAM-1. This suggests that the acute responses of sICAM-1 and sVCAM-1 are dependent upon exercise volume, and there may be an exercise intensity threshold where sICAM-1 and sVCAM-1 respond to exercise.The current study showed that sVCAM-1 significantly increased 24 hours after lower-intensity exercise and remained elevated up to 48 hours. One of the possible explanations for an increase in sVCAM-1 following exercise may be due to a shear force, a direct impact of increased exercise-induced stress on endothelial cell structure [30]. In contrast, sICAM-1 in the current study was not altered following lower-intensity resistance exercise, which might be related to the different expression of cell adhesion molecules on different tissues. For instance, sICAM-1 is mostly expressed by a host of cells, such as hematopoietic lineage and fibroblasts, while sVCAM-1 is mainly expressed by activated endothelial cells and smooth muscle cells [31]. Thus, sVCAM-1 is believed to be a more sensitive marker in response to exercise-induced stress. Interestingly, sICAM-1 in the current study decreased following higher-intensity resistance exercise. One of the possible explanations may be due to increased shear force during high-intensity exercise. High-intensity exercise may lower circulating leukocytes and soluble cell adhesion molecules due to increased shear force caused by a large increase in cardiac output. This increased cardiac output facilitates washing out of circulating leukocytes and soluble cell adhesion molecules, thereby decreasing leukocytes or cell adhesion molecules in circulation [20, 21, 32]. Additionally, the shear force is increased by increased exercise intensity [30, 33], and the magnitude of the shear force can modulate activation of sICAM-1 and sVCAM-1 [34]. Thus, the changes in sICAM-1 and sVCAM-1 following different intensities of resistance exercise may also be related to different magnitudes of the shear force, although further work is required to determine the effects of different intensities of resistance exercise on shear force-mediated changes in sICAM-1 and sVCAM-1.
5. Conclusions
- The findings of the current study suggest that higher-intensity resistance exercise does not negatively affect cell adhesion molecules, but it may help improve cardiovascular health by decreasing sICAM-1 without changing sVCAM-1. Increased sVCAM-1 following lower-intensity resistance exercise, however, should be further explored to better understand its mechanism by examining the interaction between oxidative stress, exercise-related stress hormones, shear force, etc. In addition, a future study examining the effects of chronic exercise on changes in cell adhesion molecules in different populations including patients with atherosclerosis is warranted.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML