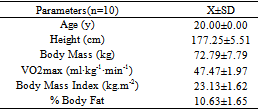-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Sports Science
p-ISSN: 2169-8759 e-ISSN: 2169-8791
2013; 3(3): 68-73
doi:10.5923/j.sports.20130303.02
Effects of Exercise on Circadian Rhythms of Cortisol
Ibrahim Erdemir1, Ali kizilet2, Tuba Kizilet Bozdogan2
1School of Physical Education and Sports, BalikesirUniversity,Balikesir, 10100, Turkey
2School of Physical Education and Sports, Marmara University, Istanbul, 34000, Turkey
Correspondence to: Ibrahim Erdemir, School of Physical Education and Sports, BalikesirUniversity,Balikesir, 10100, Turkey.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
This study was to investigate the effects of exercise on the circadian rhythm of cortisol practiced at the lowest and peak levels of cortisol circadian rhythms in 10 males (age 20 years). Measurements were taken over a four-week period. In first week, physiological parameters, body mass index and performance parameters were measured and psychological tests conducted for all participants. In second week, circadian rhythm of cortisol was determined, and blood was taken every 4-hour over 24-hour (08:00am-12:00pm-16:00pm-20:00pm-00:00am-04:00am) from each participant. In third week, participants performed exercise day-morning at 08:00am at peak level of circadian rhythm of cortisol. Blood was taken before and after each exercise day-morning, as well as every four hours over 24-hour. Finally, in the last week of study, participants performed exercise day-evening at 20:00pm at the lowest point in circadian rhythm of cortisol and their blood were also taken before and after the exercises in addition to the four hour regimen. Exercising at the lowest and peak points of cortisol changed the body’s cortisol circadian rhythm. Cortisol levels were higher after exercise day-evening than after exercise day-morning. Although the level of cortisol at exercise day-evening was higher than at exercise day-morning, recovery period after exercise day-evening was faster than after exercise day-morning.
Keywords: Morning Exercise, Evening Exercise, Hormone, Circadian Rhythm
Cite this paper: Ibrahim Erdemir, Ali kizilet, Tuba Kizilet Bozdogan, Effects of Exercise on Circadian Rhythms of Cortisol, International Journal of Sports Science, Vol. 3 No. 3, 2013, pp. 68-73. doi: 10.5923/j.sports.20130303.02.
Article Outline
1. Introduction
- The idea of circadian rhythms concept in human physical performance has been extensively researched[1, 2,3] Physical activities involving aerobic and anaerobic fitness have displayed a clear circadian rhythms[4, 5, 6]. As such, there has been great interest in trying to explain the mechanisms responsible for the distinction in exercise performance throughout the day. In humans, the principal circadian pacemaker is the suprachiasmatic nucleus (SCN). The SCN, located within the hypothalamus, gets direct input regarding the solar cycle from the retina[7] With this information provided through the retino-hypothalamic pathway, the SCN co-ordinates daily biological rhythms[8, 9]. Many physiological functions associated with athletic performance have also been shown to follow a specific circadian rhythm[10, 11]. In addition, Youngstedt and O’Connor (1999) also interpret seven other variables, that may contribute to the lack of performance in the mornings, which might help to explain the circadian rhythm in exercise performance; differences in nutritional status from morning to evening; decreased flexibility in the morning; insufficient time to recover from sleep inertia; preferred time of training; differences in the amount of rest between test sessions; individual difference in the physiological response; and differences in motivation and expectancy effect[12].Generally considered the primary catabolic hormone, cortisol is the main member of a family of steroid hormones called glucocorticoids. Corticosterone is the other glucocorticoid of interest; however, it is thought to be much less potent than cortisol, accounting for around 4–5% of total glucocorticoid activity[11]. Cortisol is synthesised and secreted from the adrenal cortex, via the hypothalamic-pituitary adrenal (HPA) axis, with a small amount also derived from the conversion of cortisone. The catabolic effects of cortisol are well known and attributed to a decrease in protein synthesis and increased protein degradation[13].The anti-anabolic properties of this hormone are also related to the attenuation of other anabolic hormones (e.g. TST and GH)[13].Chronically elevated cortisol levels have been linked to various stressors (e.g. depression, trauma, over-training); hence, this glucocorticoid is also considered one of the primary stress hormones[14]. In blood, >90% of this hormone is bound with plasma proteins, mainly with cortisol binding globulin and the rest with albumin, with the remaining fraction (<10%) circulating freely[11]. No studies have sought to directly examine changes in blood-free cortisol under exercising conditions and, therefore, only data relating to total cortisol will be discussed. Cortisol is a glucocorticoid often used as a marker of both physiological and psychological stress. Prolonged elevation of Cortisol has been shown to exhibit an inhibition effect on the neuromuscular system. Tafet et al. (2001) eplained a negative correlation between physical performance and prolonged elevated salivary Cortisol levels[15]. The circadian profile of Cortisol peaks in the morning before slowly declining throughout the day and elevating again within the first few hours of sleep[16]. Cortisol hormone and exercise performance display such distinct circadian rhythm and that they have strong implications for exercise adaptation, it is may be possible that a relationship exist between both variables despite having an inverse pattern of circadian rhythm[17, 18].It is tried to determined the reaction of the cortisol to the exercise in many studies[19, 20, 21]. Our main goal in this study is to research the effect of the exercise on the circadian rthyms of the plasma cortisol by determining the circadian rhythm of the cortisol. The plasma control compares the reaction to the control days (the days without exercise) to determine the reaction to the exercise in the early morning (the peak value of the day) and the late in the evening (the bottom value of the day) and the effect of the exercise.
2. Methods
2.1. Participants
- In this study, 20-year old 10 male university students doing irregular recreationally 2-3 days a week for recreation participated voluntarily. The participants participating in the study were kept out of the external factors that may have positive or negative effects of (such as smoking, alcohol, and drug-use). All the participants were informed about the application at the beginning of the study, the forms defining the working conditions were read and signed. The voluntary participance is necessary.
2.2. Material and Procedure
- Performance and anthropometric parameters. The length, body weight and skinfold body fat percentage (chest, abdomen, thigh, triceps, biceps, sub-scapula and Supra-iliac) (durning womersly), and the body mass index were calculated. The walk-run test (cooper) and the aerobic power (Vo2 ml/kg. min = the speed per minute (meter). 0,2 ml/kg.min) were determined in twelve minutes. Psychological measurement. The Trait Anxiety Inventory and the State Anxiety Inventory of psychologocal measurements were applied to the participants on the control day, exercise day-morning and exercise day-evening[22]. Blood Parameters. 4 ml blood samples taken form each subject by the expert health technicians were gathered in gel containing tubes and handed to the laboratory within 30 minutes by keeping at +4°C. The blood analysis were carried out at the laboratory of the Trakya University, Medical Faculty, the Department of Nuclear Medicine. The analysis of the blood data (cortisol values) were detected after 1 hour.Determination of the cortisol circadian rhythm. A total of 20 tubes of blood for determination of cortisol circadian rhythm was taken in the research. Six tubes of them were taken at 08:00–12:00–16:00–20:00–24:00–04:00 o’clock (the control day) to determine the cortisol circadian rhythm. Seven tubes of blood was taken at 08:00/09:00 (2 measurements, pre-exercise/post-exercise)–12:00–16:00– 20:00–24:00 and 04:00 o’clock (the exercise day-morning (ME)) and seven tubes of blood was taken at 20:00/21:00(2 measurements, pre-exercise/post-exercise)–24:00–04:00– 08:00–12:00 and 16:00 o’clock (the exercise day-evening (EE)).
2.3. Methodology
- It was enabled that the participants did not any heavy pyhsical exercise in the 18 hours before the test, slept and ate regularly (Breakfast: 09:00 o’clock, Midday: 12:30 o’clock, Evening: 18:00 o’clock). First week; the performance and anthropometric parameters were determined. Second week (control day); the blood was taken at 08:00–12:00–16:00–20:00–24:00–04:00 o’clock, the state-trait anxiety inventories were applied. The blood pressure and the hearth rate were detected and recorded before each measurement. The participants did not do any exercises. The third week (the exercise day-morning); 2 measurements were taken at 08:00–09:00 o’clock, as pre-exercise/post-exercise. In the mean time the state anxiety inventory was applied pre- and post-exercise. The blood was taken at12:00–16:00–20:00–24:00 and 04:00 o’clock, the trait anxiety inventory was also applied. The fourth week (the exercise day-evening); 2 measurements were taken at 20:00–21:00 o’clock, as pre-exercise/post-exercise. The state anxiety inventory was applied pre- and post-exercise. The blood was taken at 24:00–04:00–08:00–12:00 and 16:00 o’clock; the trait anxiety inventory was also applied. The blood pressure and heart rate were recorded.
2.4. Exercises
- The exercises were done on Tuesdays with a week apart at the end of December in winter. The exercise day-morning was done at 08:00 o’clock; the exercise day-evening was done at 20:00 o’clock at 0°C. The exercise program: the warmup for 25 minutes, the loadings were carried out as 4x100 meter 85%, 2x400 meter 85%, 2x800 meter 85%. The participants were done passive recreations between the exercises (HR 120)[23]. The target of 85 % hearth rate reserve for the work load was calculated as[23]Heart Rate (HR) target = HR resting + 0.85 (HR max – HR resting)(The calculated HR in exercise was approximately 180-185 bpm for all exercise).The heart rate were determined according to the recreation pulse of the participants. As the recreation heart rate were not similar, the participants were divided into two groups according to the heart rate during the exercise.
2.5. Data Analysis
- Descriptive data analysis were generated for all variables and expressed as mean ± SD. Statistical analysis involved a two-way analysis of variance (group X time) with repeated measures. Once main effects were identified, individual differences between means were determined by the Bonferroni post-hoc procedure. Pre-exercise and post-exercise difference values (pre-ME – post-ME X pre-EE – post-EE) were compared using the nonparametric Wilcoxon Signed Ranks test. Statistical significance was accepted when p<0.01 and p<0.05.
3. Results
3.1. Physical Characteristics and Demografics
- The demografics and physical characteristics of all the participantss were presented in the Table 1.
|
3.2. Psychological Parameters
- In the statistical comparisons of psychological situation were found thatthe state anxiety inventory applied to the participants “in the control day (44.10±3.79), the exercise day-morning (44.10±4.01) and exercise day-evening (43.70±4.22)” and the trait anxiety inventory applied “in the control day (45.00±3.06), exercise day-morning (44.40±3.60) and the exercise day-evening (43.80±3.8)”, any psychological state affecting the cortisol level of the participants in the application days was not detected. As a consequence, the variance analysis value was found as F2-27=0.03 in the state anxiety inventory and as F2-27=0.31 in the trait anxiety inventory, and there were not any statistical differences in the comparisons of the application days and the control day. The data obtained in the Borg scale applied to the participants during the exercise day-morning and evening was determined as “exercise day-morning” 4,50±2,01 and “exercise-day evening” 6,00±1,70. In the comparison of the Borg scale values during the exercise day-morning and evening (z=-1.62), there was not any statistical difference between the morning-exercise and the evening-exercise (Table 2).
|
3.3. The Comparison of the Cortisol Levels Determined in the Application Days (Control Day, Exercise Day-morning and Exercise Day-evening)
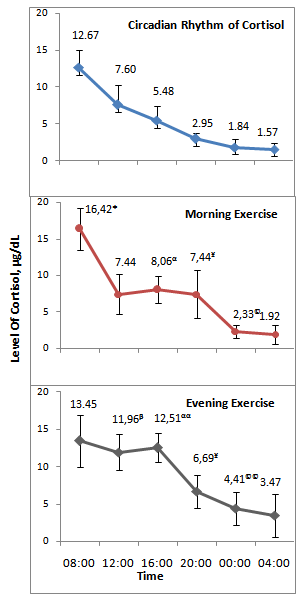
- The comparison of the cortisol levels determined in the application days (control day, exercise day-morning and exercise day-evening) were observed like that the blood cortisol levels taken on the specific hours in the application days were compared and found that the blood cortisol levels taken at 08:00 o’clock (F2-27=4.58) in the application days was considered, the differences in the p<0.05 levels were determined. The blood cortisol level of exercise day-morning was higher than that of exercise day-evening and control day(Figure 1).The significance at level of p<0.01 were determined between the blood cortisol values (F2-27=9.64) taken at 12.00 o’clock in the application days. The average of exercise day-evening blood cortisol was detected higher than the control day (mean differences-4.36µg/dL) and the exercise day-morning (mean differences-4.52µg/dL) the blood cortisol level (Figure 1). However, there were not any statistical differences between the control day and the exercise day-morning blood cortisol level. The blood cortisol level taken at 16.00 o’clock (F2-27=34.15) between the application days were determined to be statistically significant (p<0.01). The blood cortisol level at 16.00 in the exercise day-evening was found to be higher than the control day (mean differences7.03µg/dL) and the exercise day-morning ((mean differences4.45µg/dL) blood cortisol level (p<0.01). The control day was lower than the exercise day-morning blood cortisol level (mean differences-2.58µg/dL) and statistically significant differences were detected (p<0.05)(Figure 1).When the blood cortisol values at 20.00 o’clock in the application days were considered, the significant differences (F2-27=10.57) at the level of p<0.01 were detected. According to the Bonferroni test in the multiple comparisons, this significance stems from the fact that the control day blood cortisol value was lower than the exercise day-morning (mean differences-4.49µg/dL) and the exercise day-evening (mean differences-3.74µg/dL) blood cortisol value(Figure 1). Significant differences (p<0.01) were found between the days in the comparisons of the blood cortisol levels (F2-27=8.30) at 00.00 o’clock in the application days. When the details were considered with the Bonferroni test, the difference between the average of the exercise day-evening and control day blood cortisol levels were found ( p<0.01), the average of the exercise day-evening blood cortisol was found to be rather high (mean differences2.57µg/dL). At the same time, a difference (p<0.05) between the exercise day-evening and the exercise day-morning was detected. The exercise day-evening blood cortisol level was higher (mean differences2.08µg/dL) than the exercise day-morning blood cortisol level (Figure 1). There were not any statistical significance between the averages of the blood cortisol levels at 04.00 o’clock between the application days (F2-27=1.75)(Figure 1).
3.4. Blood Cortisol Levels; Morning Exercise and Evening Exercise Pre-test and Last-test
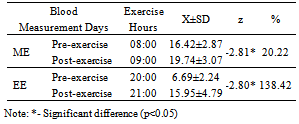
- There was a significant difference in the p<0.05 level in the comparison of the exercise day-morning blood cortisol level pre-test (08:00 o’clock) and last-test (09:00 o’clock) values (z=-2.81), in the comparison (z=-2.80) of the averages of exercise day-morning pre-test (20:00 o’clock) and last-test (21:00 o’clock) blood cortisol levels (Table 3).
|
4. Discussion
- The endocrine system, especially cortisol, always plays an important role in exercise, strength and power development by mediating the remodeling of muscle protein. Therefore, the level of cortisol changes depend on intensity, density and time of exercise; cortisol levels increase when we start to exercise. However, exercise does not affect the circadian rhythm of cortisol for a long period. After recovery period, level of blood cortisol goes back to normal. Both acute and chronic changes in blood cortisol levels were found[24]. Therefore, it can be suggested that, same research methods can be used before and after long-term training methods. The results of the study support argument made by the bulk of the existing research, that the circadian rhythms of Cortisol and blood parameters are influenced by exercise. Data extracted from Rudolph and McAuley (1998) quantitative research on cortisol levels, supported our initial hypothesis concerning the relationship between cortisol levels and exercise-induced affective states[25]. Crewther et al. (2008) investigated the salivary testosterone and cortisol levels in three different loading schemes[26]. The authors indicated that, “the similar testosterone and cortisol responses on the power and maximal strength schemes (of equal volume) support such a view and suggest that differences in load intensity, rest periods, and technique are secondary to volume.”.
5. Conclusions
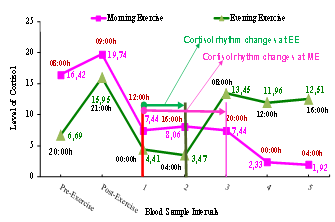
- The research shows that recovery time after exercise day-evening is faster than recovery time after exercise day-morning. Moreover, mean level of blood cortisol at exercise day-evening decreases and recovers faster, and psychological state is better at exercise day-evening than at exercise day-morning. In addition, mean level of blood cortisol changes due to exercise, especially at exercise day-evening. As a consequence, there were some changes in the exercise day-morning and exercise day-evening blood cortisol level. However, this change did not last long and after a short while, blood cortisol level returned to the circadian rhythm. Although the exercise day-evening doubled the normal blood cortisol level, it gathered itself up more quickly and returned to its old rhythm compared to the exercise day-morning. This situation shows that after the loadings in the exercise day-evening, the cortisol increase was immense and the gathering up was more quickly(Figure 2.).
5.1. Practical Implications
- •When the situation is considered, it can be said that it is more appropriate to do the high intense workout in the evening.•If trainers wants to do exercise two times in a day, recovery training, low volume and intensity, should be done at the morning.•Exercises doing high stress, strength training and speed, should be done in the evening.
AKNOWLEDGEMENTS
- The authors would like to thank to Nuclear Medicine Laboratory at the Faculty of Medicine at Trakya University for their help in data analysis process. Also special thanks is extended to the participants who devoted their time for this study. The study did not receive any financial support from any institutions. This study is conducted with the support of the ethics committee (Number: B.30.2.MAR.0.01.00.02/AEK-324)of the Marmara University
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML