-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Sports Science
p-ISSN: 2169-8759 e-ISSN: 2169-8791
2013; 3(1): 4-9
doi:10.5923/j.sports.20130301.02
ACUTE Glutamine Supplementation Does not Affect Muscle Damage Profile after Resistance Training
Bianca Ramallo1, Mario A. Charro2, Denis Foschini3, Jonato Prestes4, Tania Pithon-Curi1, AlexandreLopes Evangelista5, Charles Ricardo Lopes6, Larissa Galatti7
1Department of Physical Education, UNICSUL, Sao Paulo,03342-000, Brazil
2Department of Physical Education, UMSC, Sao Caetano do Sul, 09550-051, Brazil
3Department of Physical Education, Methodist University of São Paulo, São Paulo, 09640-000, Brazil
4University Catolic of Brasília, Program de Post-Graduation of Physical Education , São Caetano do Sul,71966-700, Brazil
5Departament Post-Graduation Fundation Antonio Prudente - Hospital of Cancer, São Paulo, 01509-900,Brazil
6Post-Graduation Program in Physical Education, Methodist University of Piracicaba, Piracicaba, SP, 13400-911, Brazil; Adventist College of Hortolandia, Hortolandia, SP, 13184-010, Brazil
7FEF-UNICAMP (Phisical Education - Campinas University), Brazil
Correspondence to: Larissa Galatti, FEF-UNICAMP (Phisical Education - Campinas University), Brazil.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Exercise-induced muscle damage occurs when untrained individuals go through strenuous and/or long-duration physical activities. Aim: to investigate the effects of glutamine supplementation on muscle damage, delayed-onset muscle soreness and muscle strength after a single exercise session in untrained individuals. Metodology: Twenty healthy male subjects with none experience in strength training in the last 12 months were selected and divided into two groups: Maltodextrine (M; n=10) and Maltodextrine plus Glutamine (MGln; n=10) and were submitted to a strength exercise session conducted with multiple sets (a method that uses more than one set per muscular group). The session was performed 72 h after the strength tests. The exercise was for horizontal shoulder adduction (bench press). The subjects performed nine sets of 6-10 maximal repetition at 75% 1-RM (maximum strength test) and rested for 1 min. ANOVA two-way was performed to compare the factors (group and time). When significant differences was indicated by ANOVA, the post-hoc Tukey HSD was performed to identify where differences occurred. In all tests, the level of significance was P ≤ 0.05. Results: Glutamine concentrations significantly increased in group MGln, the change was 36.6% in the time out set training and after training compared to 30’ before training\supplementations and 41.46% them compared with M group in the time out set training. Group M showed no significant difference in any time evaluated. (p=0.05). Conclusions: ). Thus, it can be concluded that the adopted resistance training protocol was efficient in inducing muscle damage, but, glutamine supplementation did not alter the magnitude of the damage.
Keywords: Muscle Damage, Glutamine Suplementation, Strength Training
Cite this paper: Bianca Ramallo, Mario A. Charro, Denis Foschini, Jonato Prestes, Tania Pithon-Curi, AlexandreLopes Evangelista, Charles Ricardo Lopes, Larissa Galatti, ACUTE Glutamine Supplementation Does not Affect Muscle Damage Profile after Resistance Training, International Journal of Sports Science, Vol. 3 No. 1, 2013, pp. 4-9. doi: 10.5923/j.sports.20130301.02.
Article Outline
1. Introduction
- Exercise-induced muscle damage (EIMD) occurs when untrained individuals go through strenuous and/or long-duration physical activities. The muscle damage may happen in different degrees, because it depends on types of contractions, with, muscle eccentric action, exercise type, specially high intensity strenght training (greater than 85% of 1RM), downhill running, moviment speed, interval time between sets and individual trainability , specially affecting beginners[1]. From the histological point of view, the injured muscle can be characterized by myofibril disruption, irregular structure of the lines ''Z'', disruption of sarcolemma and cytoskeleton, irregular location of organelles, and increased mitochondrial density and content of myofibrillar proteins[2].EIMD symptoms are stiffness and swelling, decreased muscular contraction force and delayed-onset muscle soreness (DOMS)[3]. DOMS can reduce the ability to perform daily tasks and athletic activities and has negative effects on adherence to physical activity programs. Therefore, it is important to determine effective prophylactic or therapeutic interventions to prevent or reduce DOMS and\or enhance muscle function recovery after strenuous exercise.It has been shown that protein and amino acid supplementation is likely to reduce muscle damage and DOMS induced by exercise. In line with[5], BCAA supplementation before and after exercise has beneficial effects when it comes to the EIMD decrease and muscle-protein synthesis. According to their findings, it is possible to consider BCAA as a useful supplement for muscle recovery. The glutamine is the most abundant amino acid in plasma and skeletal muscle[6] and accounts for more than 60% of the total intramuscular free amino acid pool[7]. Glutamine supplementation has been used by athletes during prolonged and exhaustive exercise[8]; however, its effects on strength training remain to be examined.The skeletal muscle glutamine pool is significantly reduced under certain metabolic activity[9]. After strenuous exercise, however, glutamine availability is reduced[10]. The glutamine supplementation is performed in order to minimize its presence in the plasma.Our initial hypothesis was that glutamine supplementation would prevent, at least in part, from muscle damage induced by resistance training. The aim of this study was to investigate the effects of glutamine supplementation on muscle damage, delayed-onset muscle soreness and muscle strength after a single exercise session in untrained individuals. CK and LDH activities, leukocyte count, TNF-α concentration, DOMS and muscle strength were analyzed.
2. Materials and Methods
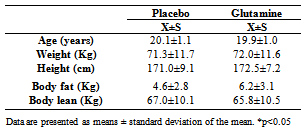
- SampleTwenty healthy male subjects with none experience in strength training in the last 12 months were selected and divided into two groups: Maltodextrine (M; n=10) and Maltodextrine plus Glutamine (MGln; n=10). The anthropometric characteristics of the participants are presented in Table 1. The experiment was approved by the Research Ethics Committee of the Methodist University of São Paulo (protocol number 0070.214.000-07). All subjects were informed in detail about the procedures to be used and voluntarily agreed to participate in the study, signing the inform term of consent.Volunteers with known history of cardiovascular and respiratory diseases, diabetes, hypertension, hormonal disorders, and muscular lesion (in the last 12 months) were excluded from the study. Individuals taking medication or supplements in the 6 months preceding the beginning of the study were also excluded.
2.1. Training Protocol
- The protocol used in this study followed the recommendations of the American College of Sports Medicine[11]. The strength exercise session was conducted with multiple sets (a method that uses more than one set per muscular group). The session was performed 72 h after the strength tests. The exercise was for horizontal shoulder adduction (bench press). The subjects performed nine sets of 6-10 maximal repetition at 75% 1-RM (maximum strength test) and rested for 1 min.In consideration to the concentric failure, and because of the fact that the subjects were sedentary the repetions were kept between 6 and 10 to ensure the exercise intensity of 75% of 1RM, specially in the last sets in accordance with the study objective.
2.2. Maximum Strength Test (1RM)
- One day after the anthropometric assessments, the one-repetition maximum (1RM) test was performed. After the warm-up (10 minutes treadmill walking at low intensity), the individuals performed a set of eight repetitions at approximately 50% of the estimated 1RM (in accordance with the load that the participants performed during the 1-RM test). Afterwards, another set of three repetitions at 70% of estimated 1RM was performed. The subsequent lifts were simple repetitions with progressively heavier loads. The test was repeated until 1RM was determined. The test was performed for all the exercises (Inclined chest fly, biceps hammer curl, inclined press, biceps preacher curl, bench press and biceps curl with bar). Two exercises were chosen per day, and these test sessions were separated by a 48 hour interval to avoid influences on determining the maximum loads (in the week of the tests, the participants did not perform any type of physical exercise). The rest interval between the sets was three minutes and the number of attempts to determine maximum load was of three according to the descriptions[12,13].
2.3. Supplementation
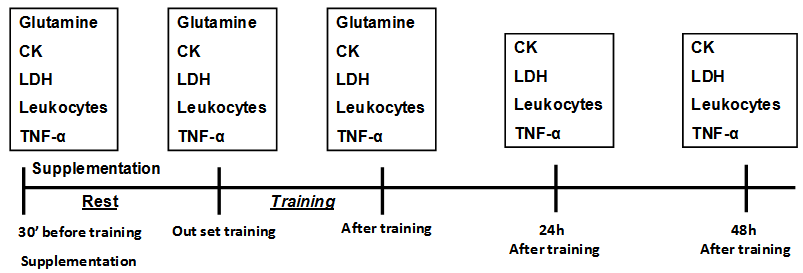
- A randomized and double blind supplementation was used. The fasted subjects ingested 50 g maltodextrin (M group) or 50 g maltodextrin plus 6 tablets of 400 mg ( 240mg glutamine dipeptide, 60mg of magnesium and 120mg glycine) (MGln group) diluted in 250 mL water. The beverages were identical in appearance and taste and prepared immediately before given to the subjects. The supplementation was ingested 30 min before exercise. Blood samples were collected from the anticubital vein before supplementation, 30 min after supplementation, and immediately, 24h and 48h after the exercise.
2.4. Blood Collections
- The first collection of 20 mL blood was carried out when the volunteers arrived at laboratory. The individuals then received glutamine supplementation and were kept at rest for 30 minutes. The same volume of blood was collected before, and immediately after the training session, 24 and 48 h afterwards. Blood was drawn from the antecubital vein into heparin-containing vacuum tubes (Becton Dickenson, St. Louis, MO). After collections, the blood was centrifuged for 10 minutes at 5000g and stored at -200C until analysis.
2.5. Glutamine Determination
- Glutamine was determined as described[14] . Production or consumption of NADH was monitored at 340 nm, using an Ultrospec 3100 pro UV/Visible spectrophotometer (Biochrom, Cambridge, England).
2.6. Determination of Activity Creatine Kinase (CK) and Lactate Dehydrogenase (LDH)
- Samples were collected for CK analyses (using kit from Sigma Chemical Co., St. Louis, MO) at the beginning and end of the training session, as well as at 24 and 48 h after the bout of strength training. The samples were processed in Cobas Mira S equipment, using thekinetic-spectrophotometric methodology and were accomplished in triplicate for quality control.
2.7. Determination of Leukocytes Number
- After homogenization was removed a portion of blood that was used to smear the blade clean with the help of blade extension. The slide was dried at room temperature (2 to 3 minutes) to perform staining with May Grünwald and Giemsa for 4 minutes. After this time, the slide was covered with 5 ml of distilled water for 2 minutes, and then washed in running water, leaving it prone to dry at room temperature. After drying, the reading was done in Neubauer chamber and determined the number of leukocytes.
2.8. Determination of TNF-α
- Plasma levels of TNF-α was determined by using enzyme-linked immunosorbent assay (ELISA), DuoSet Kit of R&D System (Quantikine, R&D System, Minneapolis, MN, USA). The detection range was of (pg/mL) 15 to 2000.
2.9. Determination of the Subjective Pain Perception
- The subjects were instructed to classify muscular pain before, immediately after the training session and, 24 and 48 h afterwards. The individuals were handed a page with a 10 cm long line that represented a subjective pain perception scale. In this visual analog scale (VAS, Visual Analog Scale), one of the extremities (0 cm) contained the verbal expression, “no pain” and the opposite extremity (10 cm) “extremely sore”[15]. The subjects had to mark a place on this line that evidenced the pain they were feeling after palpation in the region of the large pectoral, brachial biceps and brachial muscle used in the training. The distance in centimeters from extremity 0 cm to the point indicated by the subject was measured with a ruler and considered the measurement of pain.
2.9.1. Maximal Voluntary Contraction
- The maximal voluntary contraction was evaluated 48h after exercise, followed the procedures described[16].
2.9.2. Statistical Analyses
- Quantitative variables were described in the respective figures by the arithmetic mean and standard deviation. To test the normality of the distribution of the parameters determined in plasma, using the Kolmogorov-Smirnov (KS test). After confirmation of the hypothesis of normal distribution ANOVA two-way was performed to compare the factors (group and time). When significant differences was indicated by ANOVA, the post-hoc Tukey HSD was performed to identify where differences occurred. In all tests, the level of significance was P ≤ 0.05.
3. Results
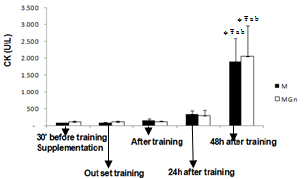
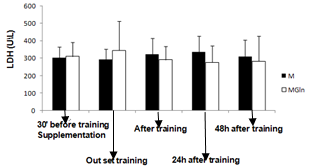
- No significant differences were observed between groups in any of the variables.
|
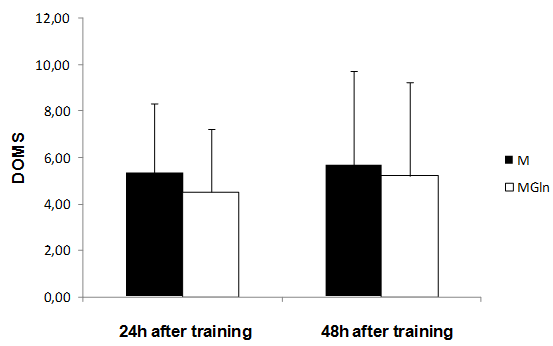 | Figure 6. Delayed onset muscle soreness (DOMS) for the supplementation (MGln) and placebo (M) group at the times: 24 and 48 hours after training. Analysis by Anova + Post Hoc Tukey |
4. Discussion
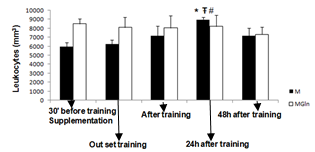
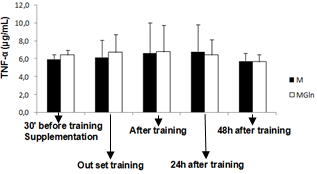
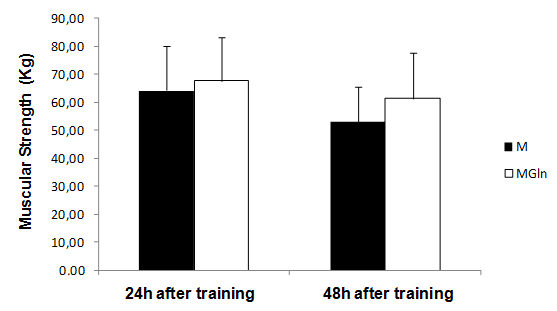
- The main finding of the present study was that the adopted resistance training protocol was efficient in inducing muscle damage, but, glutamine supplementation did not alter the magnitude of the damage. In this way, our initial hypothesis has not been confirmed, since glutamine produced no effects on muscle damage protecting.There is a paucity of studies that investigated the associated effects of glutamine supplementation with resistance training. The three main studies found no beneficial effect of glutamine supplementation in response to resistance training[17,18]. There are more studies on glutamine supplementation and exhaustive aerobic training. Some of these studies actually showed positive effects of glutamine on aerobic performance[19,20] immune function[21, 22, 23] and muscle damage protection[4].The benefic effects of glutamine on muscle damage attenuation can be partially explained by the fact that exhaustive aerobic exercise is associated with an increased oxidative stress due to the synthesis of reactive oxygen species (ROS) and reactive nitrogen species (RNS), which are also responsible by muscle damage[8]. In this sense, antioxidant systems, such as glutathione (GSH) are essential in reducing the interaction of ROS and RNS with muscle cells, which can attenuate the exhaustive exercise induced oxidative stress, decreasing in incidence of muscle damage. GSH is the most important non-enzymatic cellular antioxidant of the body[24], some of the functions of the GSH are to resist against damage and oxidative stress[24]. GSH synthesis relies on the levels of cysteine, glycine and mainly glutamate[25], so that glutamate levels is depends on the availability and transport of glutamine into the cell[25]. In the intracellular environment, glutamine can be hydrolyzed and elevate glutamate availability, favoring the synthesis of GSH and assisting in the protecting process against muscle damage. Until the moment, no studies that evaluated the interaction between glutamine supplementation, resistance training and muscle damage were found. However, a recent study[26] evaluated the effects of a supplementation (4g of isolated leucine, BCAA containing 4g of leucine, 1g of isoleucine and 1g of valine or 4g of glutamine) associated with a resistance training session on the absorption of these amino acids. The results showed that leucine and BCAA levels were lower (24% and 27%, respectively) after resistance training as compared with baseline. This suggests that, probably these amino acids were oxidized and used as an energy source to stimulate protein synthesis and reduce catabolic processes[27]. Plasma glutamine levels exhibited a similar pattern at baseline and after exercise. With the supplementation there was an increase of 14% at baseline and of 26% after resistance training[26].The reason why resistance training does not alter glutamine, but induce huge modifications on leucine, arginine e taurine plasma levels is unknown. Probably this physiological difference is associated with the difference in glutamine metabolism. According to[7,26] glutamine does not affect the performance during resistance training and its supplementation before a training session seems to be unnecessary. However, as mentioned earlier, glutamine exerts several benefic effects on the body, but probably, these effects do not relay on supplementation timing and the extracellular glutamine may not be necessary as an additional supplement for healthy people[26].These studies showed that although glutamine supplementation may be efficient during and after exhaustive aerobic training, its effects are not manifested for resistance training, since the above mentioned studies presented no effects of glutamine on protein synthesis, decrease of muscle catabolism, or aid as an energy source. Additionally, in the present study there was no protecting effect of glutamine supplementation against the muscle damage induced by resistance training.Possibly the protecting effect of glutamine on muscle damage induced by exhaustive aerobic exercise is restricted to its participation in the synthesis of antioxidants and by decreasing the formation of ROS and NRS that is more significant during exhaustive aerobic training than resistance training.In the present study glutamine supplementation induced a lower leukocytes count 24h after the training. It is difficult to explain this result, but, there is an association of glutamine with the modulation of the immune function, as already shown by previous studies as those from[23,28]. Concluded that, glutamine supplementation 30 minutes before resistance training is not efficient in preventing from muscle damage in untrained individuals, showing that this may not be a necessary procedure for healthy people.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML